Development of Medicines for Pediatric and Rare Diseases
1–3 Feb 2017, Universitäts-Kinderspital beider Basel, Switzerland
Welcome from the Chair
We would like to draw your attention to the 3rd conference ”Development of Medicines for Pediatric and Rare Diseases - Annual Event for Interdisciplinary Challenges” in Basel, Switzerland, February 2-3, 2017, preceded by a master class on commercial drug development in the morning of February 1, and a master class on Pediatric Drug development in the afternoon of February 1.
The first two conferences in 2015 & 2016 attracted more participants than we had hoped for. We are confident that even more attendees will participate in 2017. We would highly appreciate your participation and would kindly ask you to block the date in your agenda. Registration is possible from now on. A first draft agenda and further updates will be sent out in the next weeks and months.
For organisational questions please direct your questions to nadine.schrutt@ukbb.ch; for content question to Prof. Johannes van den Anker, Johannes.vandenAnker@ukbb.ch and Dr. Klaus Rose, klaus.rose@klausrose.net; for sponsoring suggestions to Martin Austin, mcaustin@transformrx.com.
Looking forward to see you in Basel, Switzerland, in February 2017
Best regards
Klaus Rose and John van den Anker
in name of the organization committee
Conference Chair
Prof. Dr. med. Johannes N. van den Anker
Chair, Department of Pediatric Pharmacology, Eckenstein-Geigy Professor of Pediatric Pharmacology, University Children’s Hospital Basel
Dr. med. Klaus Rose
Pediatric Drug Development & More, Aeussere Baselstrasse 308, 4125 Riehen, Switzerland
Programme Committee
Phil Walson, USA & Germany
Klaus Rose, Switzerland
Martin Austin, Switzerland
John van den Anker, Switzerland & USA
Marc Pfister, Switzerland
Urs Frey, Switzerland
Solange Rohou, France
Further Faculty
Phil Walson, USA & Germany
Koenraad Norga, Belgium & London
Hulya Oezsahin, SwissMedic, Switzerland
Trish Smith, Aerogen, Ireland & USA
Hubert Caron, Roche, Switzerland & USA
Adina Tokoian, Shire, Switzerland
Karin Jorga, Switzerland
Jean-Marc Nuoffer, Switzerland
Norbert Poellinger, Glatt, Germany
Martin Graham, USA
Michael Katz, USA
Conference Secretariat
Dr. Leoni Barrios
Matthias Burkhalter
Email : dmprd2017@mdpi.com
Tel. +41 61 683 77 35
Sponsoring Opportunities
For information regarding sponsoring opportunities, please contact Martin Austin.
Email : mcaustin@transformrx.com
Conference Chairs
Conference Venue
The conference will be held at the Basel University Children's Hospital. The address is Spitalstrasse 33, Basel.
Basel
Basel is the third largest city in Switzerland and is located in the Northeast of the country, on the border of France and Germany. It is an important economic center in Switzerland and offers a rich cultural life. It is home to the Museum of Fine Arts, which holds the most significant and largest collection of art in Switzerland, the Foundation Beyeler, the Theater Basel and the Musical Theater Basel.
Located on the bank of the Rhine and situated only a few kilometers from the German Black Forest and French Vosges, it also offers great possibilities for excursions in the nature.

University of Basel
The University of Basel was founded in 1460 and is the oldest university in Switzerland. It prides itself in its tradition of over five hundred years of excellence in teaching, learning, and research, during which it has adopted a forward-looking approach to new developments in science to provide high-quality education and underpin its well-deserved reputation as a university capable of attracting staff and students from all over the world. Located in the heart of Basel, and drawing on innovation anchored in tradition, the University of Basel is dynamic and innovative. Research at the University of Basel focuses on the life sciences and culture. Both core areas comprise a wide range of projects, involving research staff and scientists committed to interdisciplinary work and a cross-faculty approach to advancing the knowledge of life and the workings of culture. The University of Basel is committed to providing state-of-the-art education and to facilitating outstanding research. (Adapted from www.topuniversities.com).

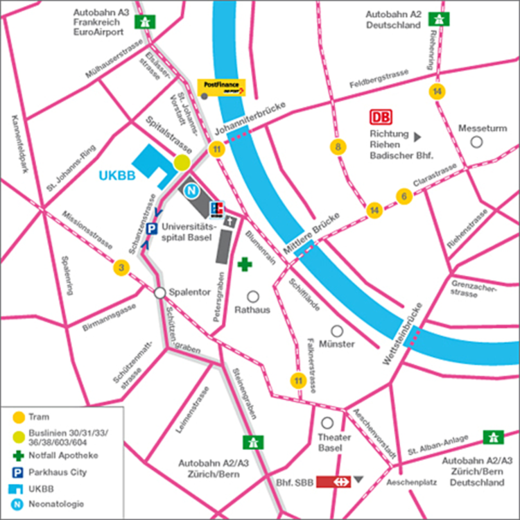
How do I get to the venue?
Congress Venue
Basel University Children's Hospital
Spitalstrasse 33
4056 Basel / Switzerland
Tel.: +41 61 704 1212
www.ukbb.ch
Public transport
Since parking is limited in the immediate vicinity of the Children’s Hospital, we would request you to use public transport if possible.
- From the SBB station, bus no. 30 to the “Kinderspital UKBB” stop.
- Buses 30, 31, 33, 36, 38, 603, 604 to the “Kinderspital UKBB” stop.
- Tram no. 11 to the “Johanniterbrücke” stop
Public transport in Basel is very reliable, with buses and trams, there is no need to hire a car for the conference.
Travelling by car
From the A2/A3 motorway:
- Either: take the exit marked Basel City/Bahnhof SBB and follow the signs to Bahnhof SBB/Universitätsspital. Use the Parkhaus City car park in Schanzenstrasse and walk to the Children's Hospital.
- Or: continue towards EuroAirport to the exit marked Basel-St. Johann; turn left at Voltaplatz into Elsässerstrasse/St. Johanns-Vorstadt, follow the signs to Universitätsspital/Kinderspital (UKBB) and park in Parkhaus City
Car parking is available in the nearby Parkhaus City or another Basel car park (charges apply).
Accommodation in Basel
Staying in Basel
Basel’s hotels offer accommodation for every taste and need. From traditional top-of-the-line hotels to youth hostels, from highly modern design hotels to the family atmosphere of a bed & breakfast, you’ll find what you’re looking for.
MasterClasses Schedule
Commercial Drug Development MasterClass
Wednesday, 1st February 2017, Basel, UKBB
Trainer: Martin Austin, Basel, Switzerland
| 8:30h | Registration & Coffee |
| 9:00h | Welcome |
| 9:10h | "Over the Rainbow" - Making medicines from ideas |
| 9:50h | Steps to successful commercialization: An Overview |
| 10:30h | Morning coffee |
| 11:00h |
Step 1: know yourself! Could you pull it through? |
| 11:30h | Step 2: Environment, Market & Competition |
| 12:00h | Step 3: Creating a Plan : The threshold criteria. |
| 12:30h | Wrap-Up |
| 13:00h | End of Masterclass |
Pediatric Drug Development MasterClass
Wednesday, 1st February 2017, Basel, UKBB
Trainer: Klaus Rose, klausrose Consulting, Basel, Switzerland
| 13.30h | Registration & Coffee |
| 14:00h | Welcome |
| 14:10h | EU & US Pediatric pharmaceutical legislation |
| 14:40h | The EU Pediatric Investigation Plan (PIP) |
| 15:30h | Childrens’ special needs in pharmaceutical treatment: developmental physiology, pediatric formulations, pediatric clinical pharmacology |
| 16:00h |
Operational challenges of pediatric clinical trials:
|
| 16:30h | Break |
| 17:00h | Impact of US & EU pediatric legislation on drug development |
| 17:30h | Interactive training : EMA pediatric website |
| 18:00h | Wrap-Up & End of Masterclass |
Publication Opportunities
Publication Opportunities
Special issue related to this conference in the journal Children.
Children (ISSN 2227-9067) is an international open access journal of pediatrics published quarterly online by MDPI. The publication focuses on sharing clinical, epidemiological, translational and basic science research relevant to children’s health.
Special Issue Information
Dear Colleagues,
The value of licensed and appropriately labeled medicines for children and adults is increasingly a focus of public awareness. This is, for a large part, the result of US and EU legislation on paediatric and orphan diseases. However, paediatric clinical practice has changed less than expected, and the initial enthusiasm has been replaced by a more realistic assessment. Breakthrough therapeutic innovations have been introduced in the last decades, e.g. for cystic fibrosis, metabolic diseases or hormonal defects. However, none of these were triggered by paediatric legislation. The aim of this special issue is to provide an update on both state-of-the-art methodology and operational challenges in research & development of paediatric and rare diseases, in order to re-evaluate what is needed for more progress in the development of treatments for both paediatric and rare diseases.
We welcome original research, review, opinion papers, editorials, or short communications on the following topics: paediatric legislation, commercial drug development, development of orphan and paediatric drugs, paediatric clinical research, developmental pharmacology, neonatal pharmacology, paediatric pharmacology, neonatal medicine, neonatal infectious diseases, pharmacokinetics, pharmacodynamics, and neonatal clinical trials.
Prof. Johannes N. van den Anker
Dr. Klaus Rose
Guest Editors
Submission
Manuscripts should be submitted online at www.mdpi.com by registering and logging in to this website. Once you are registered, click here to go to the submission form. Manuscripts can be submitted until the deadline. Papers will be published continuously (as soon as accepted) and will be listed together on the special issue website. Research articles, review articles as well as communications are invited. For planned papers, a title and short abstract (about 100 words) can be sent to the Editorial Office for announcement on this website.
Submitted manuscripts should not have been published previously, nor be under consideration for publication elsewhere (except conference proceedings papers). All manuscripts are refereed through a peer-review process. A guide for authors and other relevant information for submission of manuscripts is available on the Instructions for Authors page. Children is an international peer-reviewed Open Access quarterly journal published by MDPI.
Please visit the Instructions for Authors page before submitting a manuscript. For the first couple of issues the Article Processing Charge (APC) will be waived for well-prepared manuscripts. English correction and/or formatting fees of 250 CHF (Swiss Francs) will be charged in certain cases for those articles accepted for publication that require extensive additional formatting and/or English corrections.
Keywords
- Better Medicines for Children
- Paediatric Drug Development
- Paediatric Formulations
- Juvenile Animal Studies in Drug Development
- Age-Specific Formulations
- Paediatric Clinical Pharmacology
- Neonatal Clinical Pharmacology
- Developmental Pharmacology
- Pharmacokinetics
- Pharmacodynamics
- Neonatal Clinical Trials
- Inclusion of paediatric aspects in drug development
- Children and history of pharmacy
- Children and history of medicine
- Children and society
Sponsoring Opportunities
Email : mcaustin@transformrx.com
Travel & Registration Information
Main Conference incl. Pre-Conference Workshops 1* & 2**
Registration Fee in CHF
Industry
By 28 October 2016 - CHF 2000
29 Oct – 4 Dec 2016 - CHF 2200
From 5 Dec 2016 - CHF 2400
Academia
Regulatory Authorities
Patient Organisation
By 28 October 2016 - CHF 1000
29 Oct – 4 Dec 2016 - CHF 1100
From 5 Dec 2016 - CHF 1200
Main Conference incl. Pre-Conference Workshop 1*
Registration Fee in CHF
Industry
By 28 October 2016 - CHF 1500
29 Oct – 4 Dec 2016 - CHF 1650
From 5 Dec 2016 - CHF 1800
Academia
Regulatory Authorities
Patient Organisation
By 28 October 2016 - CHF 750
29 Oct – 4 Dec 2016 - CHF 825
From 5 Dec 2016 - CHF 900
Main Conference incl. Pre-Conference Workshop 2**
Registration Fee in CHF
Industry
By 28 October 2016 - CHF 1500
29 Oct – 4 Dec 2016 - CHF 1650
From 5 Dec 2016 - CHF 1800
Academia
Regulatory Authorities
Patient Organisation
By 28 October 2016 - CHF 750
29 Oct – 4 Dec 2016 - CHF 825
From 5 Dec 2016 - CHF 900
Conference Only
Registration Fee in CHF
Industry
By 28 October 2016 - CHF 1000
29 Oct – 4 Dec 2016 - CHF 1100
From 5 Dec 2016 - CHF 1200
Academia
Regulatory Authorities
Patient Organisation
By 28 October 2016 - CHF 500
29 Oct – 4 Dec 2016 - CHF 550
From 5 Dec 2016 - CHF 600
Workshops 1* & 2**
Registration Fee in CHF
Industry
By 28 October 2016 - CHF 1000
29 Oct – 4 Dec 2016 - CHF 1100
From 5 Dec 2016 - CHF 1200
Academia
Regulatory Authorities
Patient Organisation
By 28 October 2016 - CHF 500
29 Oct – 4 Dec 2016 - CHF 550
From 5 Dec 2016 - CHF 600
Workshop 1*
Registration Fee in CHF
Industry
By 28 October 2016 - CHF 500
29 Oct – 4 Dec 2016 - CHF 550
From 5 Dec 2016 - CHF 600
Academia
Regulatory Authorities
Patient Organisation
By 28 October 2016 - CHF 250
29 Oct – 4 Dec 2016 - CHF 275
From 5 Dec 2016 - CHF 300
Workshop 2**
Registration Fee in CHF
Industry
By 28 October 2016 - CHF 500
29 Oct – 4 Dec 2016 - CHF 550
From 5 Dec 2016 - CHF 600
Academia
Regulatory Authorities
Patient Organisation
By 28 October 2016 - CHF 250
29 Oct – 4 Dec 2016 - CHF 275
From 5 Dec 2016 - CHF 300
Networking Event Evening of February 2: Complimentary
* Pre-Conference Workshop Commercial Drug Development MasterClass (Morning of 1 February 2016)
** Pre-Conference Workshop Paediatric Drug Development MasterClass (Afternoon of 1 February 2016)Registration fees include
• Attendance to all sessions
• Conference Documents
• Lunch
• Coffee breaks
• Networking Event
Confirmation
Upon receipt of the correct registration fee, each participant will receive a confirmation of registration. Please bring this confirmation to the registration desk at the conference venue as proof of your registration.
Cancellation/Name change
Refund of fees, less 25% administrative charges, can be applied for in writing until 18 December 2016 to the Administrative Secretariat. After this date no refund will be possible. Substitutions of attendees can be made at any time. For any change of names, a fee of CHF 30 will be charged.
Students
Students have the possibility to apply for a special discount. Please send your application together with a copy of a valid student card. The rate for students is CHF 120.
Responsibility
The participant acknowledges that he/she has no right to lodge damage claims against the organizers should the holding of the congress be hindered or prevented by unexpected political or economic events or generally by force majeure, or should the non-appearance of speakers or other reasons necessitate programme changes. With registration, the participant accepts this proviso.
By finishing the application, the participant declares that he/she agrees to its personal and company data being processed by MDPI and that this data may be used for information purposes on congresses and events in related fields organized by MDPI. In the opposing case, the participant should notify MDPI at the time of returning the signed application.
For inquiries, please contact Conference Secretariat.
Conference Schedule
Third Annual Conference on Drug Development in Pediatric and Rare Diseases: Successes & Opportunities - 2/3rd February 2017 Basel, Switzerland.
Day 1 |
| Time | Activity | Speaker |
| 8:00 | Breakfast and Registration | |
| 8:45 | Welcome | Klaus Rose & John van den Anker |
| Session 1: Success Stories 1 - Pediatric Drug Development. Moderation: Swissmedic & Koenrad Norga | ||
| 9:00 | Pediatric Drug Development | Adina Tokojan |
| 9:30 | Update on the March of Dimes | Michael Katz |
| 10:00 | ||
| 10:30 | Coffee Break | |
| 11:00 | Pediatric Drug Development | Hubert Caron |
| 11:30 | Neonatal Pulmonary Drug Delivery Devices | Trish Smith |
| 12:00 | Hands-on diagnosis & treatment of rare diseases | Jean-Marc Nuoffer |
| 12:30 | Lunch Break & Exhibitors Hall Visit | |
| Session 2: Regulatory Challenges. Moderation: Phil Walson & Michael Katz | ||
| 13:30 | EMA/PDCO perspective | Koenraad Norga |
| 14:00 | Pediatric perspectives in drug development: Switzerland | Hulya Oezsahin |
| 14:30 | Industry Perspective | Solange Rohou |
| 15:00 | Adopting the last therapeutic orphans: an update on the latest developments | John van den Anker |
| 15:30 | Discussion | |
| 16:30 | Refreshments for die-hards and networking | |
| 18:00 | Come Together | |
Day 2
|
| Time | Activity | Speaker |
| 8:45 | Welcome | Klaus Rose & John van den Anker |
| Session 3: Regulatory and scientific challenges - Strategic Thoughts. Moderation: Martin Austin | ||
| 9:00 | From research to bedside: academic drug development- reality, promise, successes | Phil Walson |
| 9:30 | What regulatory authorities do in pediatric drug development – and what they should do | Klaus Rose |
| 10:00 | Coffee Break | |
| 10:30 | Demonisation of commercial drug development: why more entrepreneurs are needed and how to do it | Martin Austin |
| 11:00 | Round Table Discussion | |
| 12:30 | Lunch Break & Exhibitors Hall Visit | |
| Session 4: Technical Advances & Challenges. Moderation: Marc Pfister | ||
| 13:30 | Pediatric Formulations | Norbert Poellinger |
| 14:00 | Scaling Models in pediatric PK/PD | Martin Graham |
| 14:30 | Bottom-up meets top-down: Complementary PBPK and PopPK modeling for regulatory approval of a dosing algorithm of valganciclovir in very young children. | Karin Jorga |
| 15:00 | Wrap-up | |
| 16:30 | End of day 2 | |
The conference will be preceded by a half day Masterclass on Commercial Drug Development by Martin Austin (morning of February 1) and by a Masterclass on Pediatric Drug Development by Klaus Rose (afternoon of February 1).