
First Canadian Peptide and Protein Community Virtual Symposium
27–28 May 2021
proteins, peptide science, Medicinal Chemistry
- Go to the Sessions
- Event Details
The First Canadian Peptide and Protein Community Virtual Symposium starts from today. Welcome to share your academic perspectives, and join the discussion.
The two-day live sessions are available here.
List of accepted submissions (41)
| Id | Title | Authors | Poster PDF | ||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sciforum-045175 | Bioinspired Interactions of Zn (II) and Cu (II) with the Antimicrobial Peptide Holothuroidin: Discovering the Action Mechanism |
Nikolas Tsaggari ,
,
|

|
Show Abstract |
|||||||||||||||||||||||||||||||||||||
|
The dramatic increase in antimicrobial resistance has led to active research for new treatments. Antimicrobial peptides (AMPs) are small 7 to 100 amino acids [1, 2]. One discovered mode of action of AMPs is membrane disruptive activity, associated with the interaction with membrane phospholipids [3]. Another way of action of AMPs is intracellular targeting. Some AMPs can cross the cell barriers and reach targets in the cytoplasm [2]. For some AMPs, the occurrence of bivalent metal ions (such as Zn2+ and Cu2+) affects their activity or mode of action either because of binding metal ions, so that microbes cannot get enough nutrients essential for their life and virulence [4] or because AMPs need the metal ion as a booster of their antimicrobial activity, affecting the charge or the structure of the peptide [5]. Here, we discuss the action mechanism of Holothuroidin I (HLGHHALDHLLK) [6], a natural derived peptide from Mediterranean Sea cucumber Holothuria tubulosa, of the interactions with important metal ions located in mammals (such as Zn2+ and Cu2+). REFERENCES |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-045329 |
MALDI/MS as a Tool to Rapidly Screen Peptide Libraries for Novel Substrates of Farnesyltransferase |
,
Emily Hildebrandt ,
Sadie Novak ,
Walter Schmidt ,
,
|

|
Show Abstract |
|||||||||||||||||||||||||||||||||||||
|
Protein farnesylation is a post-translational modification where a 15 carbon farnesyl isoprenoid is appended to the C-terminal end of a protein by Farnesyltransferase (FTase). In the canonical understanding of FTase, the isoprenoids are attached to the Cysteine residue of a four amino acid CaaX box sequence. However, recent work has shown that five amino acid sequences can be recognized, such as the pentapeptide CMIIM. This new discovery greatly increases the number of potential FTase substrates, as the FTase is already known to tolerate a wide variety of amino acids in the canonical CaaX sequence. Due to the large number of potential substrates it is difficult to assay for novel CaaX sequences. With the goal of developing a more rapid and methodical method to evaluate potential substrates, we envisioned using MALDI to assay libraries of 10 peptides at a time, varying one amino acid in the CaaX box to all 20 canonical amino acids over two libraries. Through this method we observed 30 hits in the mass spectrum and chose eight for further evaluation. Seven of these sequences are novel substrates for FTase, with several meeting or surpassing the in vitro efficiency of the benchmark sequence CMIIM. Additionally, in vivo experiments in yeast demonstrate that proteins bearing these sequences can be efficiently prenylated in a biological context. |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-045332 |
N-aminoimidazole-2-ones Peptide Mimics Synthesis and Applications , Pradeep Chauhan ,
Suresh Vutla ,
Julien Poupart ,
Mukandila Mulumba ,
,
Submitted: 30 Mar 2021 Abstract: Show Abstract |
,
Pradeep Chauhan ,
Suresh Vutla ,
Julien Poupart ,
Mukandila Mulumba ,
,
|

|
Show Abstract |
|||||||||||||||||||||||||||||||||||||
|
Peptide secondary structures have privileged roles in molecular recognition and therapeutic potential. α-Amino lactam residues have been commonly used as conformational constraints to study peptide structure-activity relationship for drug discovery [1]. N-Aminoimidazolone (Nai) residues offer similar means for constraining peptide backbone geometry [1, 2]. In model peptides, (4-methyl)Nai residues were found to adopt the central position of β- and γ-turn secondary structures. The addition of substituents at the 4- and 5-positions of the Nai residues may be used to mimic side chain function and orientation [3, 4]. Our presentation will feature the synthesis and application of Nai residues in the study of peptide structure-activity relationships [5].
|
|||||||||||||||||||||||||||||||||||||||||
| sciforum-045374 |
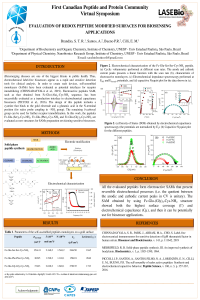
Evaluation of Redox Peptide Modified Surfaces for Biosensing Applications |
,
,
Júlia Piccoli ,
Paulo Bueno ,
|
|
Show Abstract |
|||||||||||||||||||||||||||||||||||||
|
Peptides are promising tools for designing sensitive and stable biosensors. For example, the redox self-assembled monolayer (SAM) based on the sequence Fc-Glu-(Ala)2-Cys-NH2 was successful evaluated as transducing interface in electrochemical biosensors. The design of such peptides includes: 1) cysteine to bind covalently the peptide to the gold electrode; 2) glutamic acid in N-terminal position to bind the ferrocene (Fc) in the amine group, and the antibody in the δ-carboxyl group, and 3) alanine to form a hydrophobic layer. Herein, we present the solid-phase synthesis of three different peptides with structure Fc-Glu-(X)2-Cys-NH2 (X=Ser, Gly or Phe) and the electrochemical behavior of the obtained SAMs. The Gly was chosen because of its smallest side chain, while Ser and Phe present hydroxyl groups (for H-bonds) and aromatic (for π-π interaction), respectively. The synthesis successful of the HPLC purified peptides were confirmed by mass spectroscopy. From cyclic voltammetry and impedance-derived electrochemical capacitance spectroscopy results, all peptides present reversible redox processes, and electron transfer rates (kET ) ranging from 17 to 31 s-1. Since the peptide with Gly residues presented both the highest surface coverage (Γ = 2.6x10-10 mol/cm2) and electrochemical capacitance (Cµ = 270 µF/cm2) values, it can be potentially applied for biosensors designing. |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-045409 |
Bio-Incorporation of TePhe, a Tellurium-Containing Phenylalanine Analogue, Preserves Protein Structure and Stability |
,
Mark Nitz
|

|
Show Abstract |
|||||||||||||||||||||||||||||||||||||
|
The heavy chalcogen tellurium (52Te) is a versatile element with many potential applications in chemical biology and biochemistry, including mass cytometry, fluorescence imaging, and protein structure determination. Using L-tellurienylalanine (TePhe), a mimic of the natural amino acid L-phenylalanine (Phe) in which the phenyl side chain is replaced by a nearly isosteric tellurophene ring, tellurium can be covalently incorporated into the proteome of prokaryotes and eukaryotes by endogenous translation machinery. Our goal is to generate proteins with near stoichiometric levels of Phe to TePhe substitutions, verify preservation of protein structure and activity upon TePhe incorporation, and ultimately exploit the site-specific tellurium centres as handles for crystallographic phasing, protein NMR spectroscopy, and bio-orthogonal reactivity. Here we report conditions for the expression of TePhe containing proteins in a standard E. coli expression system and validate the ability of TePhe to act as an effective Phe analogue within a folded protein. Our target for TePhe incorporation is the streptococcal immunoglobulin-binding Protein G B1 domain (GB1), a remarkably heat-stable 56-residue domain containing 2 Phe residues which pack against one another within the domain’s hydrophobic core. In Phe-deficient media containing glyphosate as an inhibitor of aromatic amino acid biosynthesis, we obtained a GB1 mixture in which approximately 1 in 2 Phe sites were substituted by TePhe. Fractionation by reverse-phase HPLC allowed us to obtain a sample with 85% TePhe substitution as evidenced by amino acid analysis. Using 1H-15N HSQC and circular dichroism spectroscopy, we find that TePhe effectively takes on the role of Phe, and alters the melting temperature of the protein by less than 5 °C. |
|||||||||||||||||||||||||||||||||||||||||
Welcome from the Chairs
Peptides and proteins are remarkable natural biopolymers, which inspire various fields of contemporary research. Moreover, they serve as components of a variety of medicines, catalysts, materials, cosmetics, and agricultural products. Our virtual symposium brings together leading researchers at the cutting edge of peptide and protein science. With a broad focus and the intent to promote the visibility of emerging scientists and young investigators, this two-day event will feature leaders in the characterization, synthesis, and application of peptides and proteins in various domains that have been targeted to improve our basic knowledge and to enhance our quality of life.
Conference SecretariatMr. Vincent Pang
Ms. Betsy Feng
Ms. Celia Xu
email: cppc2021@mdpi.com
Live Sessions
Date: 27-28 May 2021


Important information for the live conference
The CPPC 2021 conference will take place in Zoom. Please check the conference program above for a detailed schedule. Out of respect to the speakers, we would highly appreciate it if you could keep your microphone muted and your camera turned off when it is not your turn to talk. If you have any questions for our speakers, you will be able to raise your hand. You will then be called upon by the moderator to ask your question.
Posters will be displayed by their authors in different breakout rooms. Please note that in order to jump from one room to the other, you will need the latest Zoom update. Here is the link to download the latest Zoom if necessary: https://zoom.us/support/download. Each of you will be responsible for jumping from room to room, so please make sure your version of Zoom allows for this feature.
Note:
Different posters will be displayed in two days.
Thursday: All: Chemical-Biology and Medicinal Chemistry (31)
Friday: All: Materials Science and Catalysis (1) All: Peptide and Protein Applications in Cosmetic Agricultural and High-tech Products (13) All: Structural Biology and Computational Methods (9) All: Synthetic and Mimetic Methods (9)
Conference Chairs

Département de chimie, Université de Montréal

Faculty of Pharmacy, Université Laval and Centre de recherche du CHU de Québec

Department of Chemical and Physical Sciences, University of Toronto Mississauga, and Departments of Chemistry and Cell & Systems Biology, University of Toronto

Western University Sr. Scientist, London Regional Cancer Program

Department of Chemistry, University of British Columbia

Department of Chemistry, University of Toronto
Invited Speakers

Department of Chemistry and Faculty of Sustainable Design Engineering, University of Prince Edward Island
Title: Stimuli Responsive, Antimicrobial and Immunomodulatory Properties of Peptide Based Materials
Antimicrobial peptides are a diverse class of cationic and amphipathic peptides that display a broad-spectrum antimicrobial activity against gram positive and gram-negative bacteria and play a key role in innate immunity of multicellular organisms. With growing concerns over the development of antibiotic resistance microbial strains, antimicrobial peptides are much studied alternative in research. This talk mainly focuses on the synthesis of chimeric peptides, and their nanoparticles and evaluation of their antimicrobial and anti-inflammatory properties. The synthesis of peptides-based materials (polymer peptide hybrids and microparticles) and their role as stimuli responsive and drug delivery carriers will also be discussed.

Department of Chemistry, Université du Québec à Montréal, Montreal, Canada
Title: Peptide-based amyloid nanostructures: from mechanisms of self-assembly to synthetic nanovaccines
Endogenous proteins are known for their ability to self-associate in living organisms into supramolecular structures that perform key physiological functions. Over the last two decades, amyloid fibrils that accomplish essential biological activities have been identified in almost all species, from bacteria to mammals. Amyloids are organized proteinaceous assemblies characterized by a cross-beta-sheet quaternary structure. The physico-chemical and biological properties of amyloids suggest that they hold great potential as biomaterials for medical applications. However, the usage of amyloids is still today limited by a number of issues, which include; (i) incomplete understanding of the mechanisms of self-assembly, (ii) challenge of controlling aggregation and self-assembly, (iii) difficulty of predicting the final supramolecular architecture from the primary sequence and (iv) potential toxicity of conformational intermediates. In this context, our research group aims at developing (bio)chemical approaches to control amyloid assembly and design functionalized peptide-based nanostructures. By using the islet amyloid polypeptide, whose deposition in the pancreatic islets is associated with type II diabetes, we are investigating the early steps of amyloidogenesis and the relation between quaternary structure and cellular toxicity. Besides, we are developing chemical strategies to modulate the (supra)molecular architecture of peptide assemblies in order to design novel synthetic nanovaccines against influenza.

Department of Chemical and Materials Engineering, Concordia University, Montreal, QC, Canada
Title: Cancer Activating Mutation in STAT5B: Elucidating the Impact on Protein Structure and Dynamics Using Atomistic Molecular Simulations
The STAT (signal transducer and activator of transcription) protein family is an important therapeutic target in leukemia and lymphoma. However, the lack of structural and dynamic information on STAT proteins limit drug design efforts. Two cancer activating mutations located in the SH2 domain of STAT5B, N642H and Y665F, are observed clinically and the molecular basis for their increased oncogenicity is not currently known. Additionally, patients with the N642H mutation are reported to have increased drug resistance and poor response to chemotherapy [1]. Here, we used molecular dynamics simulations to elucidate the dynamics of the wild type and oncogenic mutants of human STAT5B protein, and to provide a molecular basis for the increased oncogenicity. We carried out extensive atomistic simulations of the wild type and mutant STAT5B proteins. The N642H mutation (i) led to a more rigid SH2 domain; (ii) significantly affected the size and dynamics of the peptide binding pockets; and (iii) increased the intra-SH2 domain interactions. Analysis of the impact of the Y665F mutation on the SH2 domain will identify the shared or different characteristics of the two mutations. The structural and dynamic information uncovered in this work may facilitate the design of small molecule drugs targeting the cancer activating mutants of STAT5B.

Department of Chemical and Physical Sciences, University of Toronto Mississauga,
Departments of Chemistry and Cell & Systems Biology, University of Toronto
Title: The effect of regulatory interactions and disease mutations in the NBDs of SUR proteins, the regulatory subunits of K(ATP) channels
ATP sensitive potassium (KATP) channels are found in the pancreas, brain, and cardiovascular system. KATP channels are of vast medical importance, as mutations in K-ATP channels causes cardiovascular disease, neonatal diabetes, hyperinsulinism, or epilepsy. KATP channels consist of four pore-forming Kir6.2 proteins and four regulatory sulphonylurea receptor (SUR) proteins. Recent high-resolution structures of the pancreatic KATP channel provide insights into the mechanism of pore closing, but not pore opening, which involves MgATP binding and hydrolysis at the nucleotide binding domains (NBDs). Further, structural information is lacking for some of the regulatory regions in the SUR protein, which are also sites of disease-causing mutations and/or phosphorylation that regulate channel opening. Thus, additional structural studies are necessary to determine how the action of the NBDs regulates channel gating and the molecular basis by which mutations cause diseases. Using nuclear magnetic resonance, CD, and fluorescence spectroscopies, we have characterized structural changes in the SUR NBDs and regulatory regions imparted by disease-causing mutations and/or phosphorylation. Phosphorylation alters the structure of a regulatory linker and its interactions with the NBDs. NBDs bearing disease-causing mutations have altered binding to the membrane-spanning domains, even when the disease-causing mutation is not at the NBD/membrane domain interface, suggesting that mutations likely disrupt allosteric pathways linking the action of the NBDs to the membranespanning domains and ultimately KATP channel opening. Thus, our data shed light on the underlying molecular basis by which KATP channels are regulated by phosphorylation and how several SUR mutations cause disease.

Schulich Faculty of Chemistry, Technion - Israel Institute of Technology, Haifa, Israel
Title: Peptoids as Versatile, Efficient and Selective Bio-Inspired Catalysts
Enzymatic catalysis is largely based on cooperativity between an active center and functional organic molecules located at its surrounding folds. High efficiency and selectivity are attributed to catalytic pockets within the structure of the proteins. Inspired by this concept, we design peptoid-based intramolecular catalytic systems in which the catalyst(s) and non-catalytic functional or structural groups are tethered in close proximity to each other, leading to highly efficient and/or selective catalytic systems for various important transformations. Peptoids, N-substituted glycine oligomers, can be efficiently generated by a solid-phase method that enables the incorporation of innumerable functional groups at specified N-positions along their spine, are stable towards different pH and oxidative conditions, and thus should be inert to catalytic transformations. In my talk I will demonstrate that peptoids can be used to facilitate cooperativity between several groups placed on one scaffold. I will show how we utilized this approach to design peptoids that catalyze (i) the oxidation of various benzylic, allylic and less activated aliphatic primary alcohols,1 (ii) the oxidative coupling of such alcohols with amines2,3 and (iii) the electrochemical oxidation of water,4,5 all with high conversions and low catalyst loadings via intramolecular cooperativity. I will also describe the cooperativity between non-selective catalysts embedded within peptoid sequences and the secondary structure of these peptoids and show how careful design of structure-function relationships leads to peptoids that catalyze (i) the oxidative kinetic resolution of alcohols,6 and (ii) the Michael addition reaction,7 both with high enentioselectivity.
gm92@technion.ac.il

Title: Tumor targeted Immune Cell Agonists (TICAs™) are fully synthetic, modular and tunable anti-cancer peptide therapeutics
Bicycles® are bicyclic peptides constrained via a chemical scaffold, which confers structural stability leading to high affinity and selectivity. Using phage display Bicycles have been discovered to both tumor cell and immune cell targets and using synthetic chemistry have been affinity optimized and assembled in a modular fashion to generate tumor targeted immune cell agonists (TICAsTM). These bispecific engagers simultaneously bind to overexpressed cell-surface targets on tumor cells (e.g. EphA2 or Nectin-4) and costimulatory receptors on immune cells (e.g. CD137 and OX40). This interaction leads to highly precise activation of immune cells in vitro, only in the presence of target positive tumor cells, and results in the secretion of pro-inflammatory cytokine such as IL2 and IFNg. In vivo, dosing of TICAs activates immune cells in the tumors of mice, resulting in potent anti-cancer immunity despite relatively short plasma exposures. TICAs represent a new generation of fully synthetic peptide-based immune modulatory anti-cancer agents.
kevin.mcdonnell@bicycletx.com

Title: Peptide ligations from tunable N-aryl peptide precursors
Chemoselective reactions that occur under mild conditions are required to synthesize and probe biomolecules in aqueous settings. To that end, ligation of -nucleophiles with -oxo-aldehydes or ketones have been pursued; however, addition of superstoichiometric amounts of aniline are typically required to construct oxime and hydrazone bonds at neutral pH via formation of an aniline Schiff-base intermediate. Moreover, while starting from ketone substrates instead of aldehydes would allow substitution at the site of ligation, synthetic challenges to access ketone derivatives from common amino acid building blocks and their slow reactivity in condensation reactions have precluded their widespread use in peptide ligations. Here, we expand the utility of oxime and hydrazone ligation reactions by providing direct access to reactive -imino amide intermediates from a site-selective, aerobic oxidation of N-aryl peptides. We demonstrate that the reactivity of N-aryl peptides can be modulated by the electronics of the aryl ring, and that various substitution at the -carbon can be introduced at the site of ligation in high yield. Efforts towards streamlining N-aryl peptide synthesis, as well as controlling E/Z ratios in ketoxime and kethydrazone peptides will also be discussed.

Department of Chemistry, University of California Berkeley
Title: Coiled coil control of EGFR trafficking, signaling, lifetime, and cell fate
EGFR exhibits biased signaling, whereby growth factor or mutation-dependent changes in receptor conformation and/or dynamics elicit distinct intracellular outcomes. We report that many intracellular EGFR outcomes are controlled by a two-state coiled coil switch located within the juxtamembrane segment (JM), an essential component of the cytosolic dimer interface. The position of this switch defines the path of endocytic trafficking, the extent and dynamics of autophosphorylation, c-Cbl recruitment, and ubiquitination, and whether or not EGFR is degraded within lysosomes. It also predicts kinase-independent effects of oncogenic mutations and clinically relevant tyrosine kinase inhibitors (TKIs) that promote lysosome-based degradation. These findings provide a model for biased EGFR signaling, insights into kinase-independent activities of EGFR and clinically relevant TKIs, and identify new strategies for modulating protein lifetime.

Title: Strategies to Mitigate Host Defense Peptide (HDP) Toxicity
Host defense peptides (HDPs) have been the subject of great interest for the treatment of multidrug resistant bacterial infection due to their multimodal activity and low induction of resistance. However, concerns regarding aggregation, toxicity and short biological half-life have limited their applicability for clinical treatment. Many methods have been explored to improve these characteristics, such as polymer (e.g., polyethylene glycol (PEG) or hyperbranched polyglycerol (HPG)) conjugation, but these are often accompanied by reductions in the activity of the HDP. Here, we detail the design of novel conjugates with improved biocompatibility. In addition, a novel conjugate that incorporates an enzymatic cleavage sequence targeting matrix metalloproteinases (MMPs) that accumulate at sites of inflammation and infection will be presented.
Instructions for Authors
Registration for this conference is FREE.
Submissions will be accepted online. Authors may submit their work by registering at www.sciforum.net and using the "Start New Submission" function once they are logged into the system. Please make other comments about contributing author’s status: post-doc, graduate student, or other in the process of submission.
- Scholars interested in participating in the conference can submit their abstract (200-word limit) on this website until 22 April 2021.
- The Chairs will pre-evaluate, based on the submitted abstract, whether a contribution will be included in the First Canadian Peptide and Protein Community Virtual Symposium. Authors will be notified by 28 April 2021 about the acceptance decision regarding their abstract.
- If the abstract is accepted, the author will be invited to prepare a PPT (oral presentation) or poster presentation by the submission deadline of 10 May 2021.
The open access journal Biomedicines will publish a Special Issue "Selected Papers from the Canadian Peptide and Protein Community" on the conference. After the conference, authors can submit the full papers to the aforementioned Special Issue (the submissions process will follow the usual procedure of the journal, including peer review, APC, etc.) with a special APC discount.
Presentation Slides
Authors are encouraged to prepare a presentation in PowerPoint or similar software, to be displayed online along with the manuscript. Slides, if available, will be directly displayed on the website using Sciforum.net’s proprietary slides viewer. Slides can be prepared in exactly the same way as for any traditional conference where research results can be presented. Slides should be converted to the PDF format before submission so that our process can easily and automatically convert them for online display.
CPPC oral presentation ppt template.pptx
Presentation of Posters
Posters will be available on this website during and after the event. As with papers presented at conferences, participants will be able to ask questions and make comments about the posters. Posters will be available online on this website during and after the virtual conference.
Posters should include the following:
• Title (with authors and affiliations);
• Introduction/objectives/aims;
• Methods;
• Results;
• Conclusion;
• References;
• Acknowledgments;
• Contact information.
There are no uniform format requirements for posters, which can be prepared in vertical or horizontal orientation.
NOTE: Uploading a full manuscript is optional, not mandatory. In the system, "manuscript PDF" is showing to be mandatory, which means as selected oral/poster presentation, you should prepare your PPT/poster in a PDF format and upload it there.
Potential Conflicts of Interest
It is the authors’ responsibility to identify and declare any personal circumstances or interests that may be perceived as inappropriately influencing the representation or interpretation of clinical research. If there are no conflicts, please state here “The authors declare no conflicts of interest”. This should be conveyed in a separate “Conflicts of Interest” statement preceding the “Acknowledgments” and “References” sections at the end of the manuscript. Financial support for the study must be fully disclosed under the “Acknowledgments” section.
Copyright
MDPI, the publisher of the Sciforum.net platform, is an open access publisher. We believe that authors should retain the copyright to their scholarly works. Hence, by submitting an abstarct/poster or other files to this conference, you retain the copyright of your paper, but you grant MDPI the non-exclusive right to publish this paper online on the Sciforum.net platform. This means you can easily submit your paper to any scientific journal at a later stage and transfer the copyright to its publisher (if required by that publisher).
Event Awards
To acknowledge the support of the conference’s esteemed authors and recognize their outstanding scientific accomplishments in the field of biomolecular and molecular science, we are pleased to launch the Best Oral Presentation Awards and the Best Poster Presentation Awards, sponsored by Biomedicines and International Journal of Molecular Sciences. Eight winners will be selected, and each winner will receive a cash award of USD 100. Winners will be announced at the end of the live session.


The Awards
Number of Awards Available: 4
The prize for each of the awards will consist of USD 100 (two will be awarded to graduate students and two to post-doctoral fellows).Number of Awards Available: 4
The prize for each of the awards will consist of USD 100 (two will be awarded to graduate students and two to post-doctoral fellows).Terms and Conditions:
- If the short abstract is accepted, PPT or posters must be submitted.
- Originality / Novelty of the paper
- Significance of Content
- Scientific Soundness
- Interest to the readers
- English language and style
Award Winners Announcement
Winners Announced—CPPC2021 Best Oral Presentation Awards & Best Poster Presentation Awards
We are so pleased to announce that the winners of CPPC2021 Best Oral Presentation Awards and the Best Poster Presentation Awards have been selected by the Conference Committee. A total of eight papers have been shortlisted. The winner will receive a 100 USD bonus. Please join us in congratulating them!
Best Oral Presentation Awards
Speaker: Sorina Chiorean
Synergistic Peptides Expand the Activity Scope of Antimicrobial Peptide Teixobactin
By Sorina Chiorean, Isaac Antwi, Daniel Carney, Antoine Henninot, and John Vederas
Speaker: Sebastian Morales
Fluorescence fluctuation imaging analysis reveals LDL and PCSK9 regulation of LDL-receptor dynamics on the cell membrane
By Sebastian Morales, Jacob Pollard, Janice Mayne, Daniel Figeys, and Paul Wiseman
Speaker: Leah Helton
Allosteric Inhibition of LRRK2 with a Helix-Turn-Helix Stapled Peptide
By Leah Helton, and Eileen Kennedy
Speaker: Arunika Ekanayake
Genetically Encoded Fragment-Based Discovery (GE-FBD) from Phage-Displayed Macrocyclic Libraries with Genetically Encoded Unnatural Pharmacophores
By Arunika Ekanayake, and Ratmir Derda
Best Poster Presentation Awards
Controlling the Self-Assembly of the Protein Flagellin into Tailored Nanostructures to Modulate its Immunostimulating Properties
By Mélanie Côté-Cyr, Ximena Zottig, Denis Archambault, and Steve Bourgault
Expanded Toolbox for Directing the Biosynthesis of Macrocyclic Peptides in Bacterial Cells
By Jacob Iannuzzelli, and Rudi Fasan
Structural Studies of Ycf1p Provide Molecular-level Insights into how Post-Translational Modifications Regulate the Activity of ATP Binding Cassette Proteins
By Sarah Bickers, Samir Benlekbir, John Rubinstein, and Voula Kanelis
By Alla Pryyma, Shanal Gunasekera, Joshua Lewin, David Perrin
The Awards
Best Oral Presentation Awards
The award will consist of 100 USD
Best Poster Presentation Awards
The award will consist of 100 USD
Honorable Mentions for Best Poster Presentation
Effects Of The Cowpea Gln-Asp-Phe Peptide Daily Administration in Rats Fed A Saturated High-Fat DietBy Mariana Barros de Cerqueira e Silva, Jaff Ribeiro da Silva, Maria Carolina Oliveira de Arruda Brasil, Biane Oliveira Philadelpho, Victória Cruz de Souza, Johnnie Elton Machado dos Santos, Rodrigo Molini Leão, Ricardo David Couto, Francine Johansson Azeredo, Marcelo Santos Castilho, Ederlan de Souza Ferreira, and Eduardo Maffud Cilli
Synthesis of Lactam Modulators of The Interleukin-1 Receptor For Delaying Labor And Improving Neonatal Outcomes
By Charity Yongo-Luwawa, Christiane Quiniou, Sylvain Chemtob, and William Lubell
Convergent Total Synthesis of Yaku'amide A and its Simplified Analogs
By Concordia Lo, and Steven Castle
Functional Characterization of Crocodylian Cathelicidins
By Felix Santana, Morgan Alford, Bing (Catherine) Wu, Evan Haney, Karel Estrada, Noushin Akhoundsadegh, Gerardo Corzo, and Robert Hancock
N-Aminoimidazole-2-ones peptide mimics synthesis and applications
By Yousra Hamdane, Pradeep Chauhan, Suresh Vutla, Julien Poupart, Mukandila Mulumba, Huy Ong, and William Lubell
A. Chemical-Biology and Medicinal Chemistry
B. Materials Science and Catalysis
Show all published submissions (1) Hide published submissions (1)
Submissions
List of Papers (1) Toggle list























