
The 1st International Electronic Conference on Vaccines
RNA Vaccines, Current Challenges and Future Developments
Part of the International Electronic Conference on Vaccines series
1–15 December 2023
Immunological mechanisms, Viral Immunology, Immunopathogenesis, vaccine development and efficacy evaluation, immune responses to vaccines, vaccine technology, vaccine vectors adjuvants and immunomodulators, Immunomodulation, Prophylactic vaccines
- Go to the Sessions
- Event Details
Live Sessions Information
During the duration of the conference, TWO live online sessions will be programmed. The live streaming platform we are using is Zoom. During each session, the participants will have the possibility to ask questions during a Q&A session. Detailed information about the topics and dates will be shared soon.
The live sessions are free of charge. The authors who submit submissions to IECV 2023 will have priority for the registration (with no extra cost) to the live online sessions with our keynote speakers. If it is not completely full, registration will be open for unregistered participants. Registrations with academic institutional email addresses will be prioritized. The number of participants in the live session is limited but the recording will be made available on Sciforum shortly afterward.
We are pleased to invite you to subscribe to our conference so that you can receive email notifications when the live session program is online and open online discussions.
Live sessions
|
CET |
EST |
CST Asia |
Speaker |
|
09:00 - 09:10 |
3:00-3:10 | 16:00-16:10 | Prof. Dr. Silvio Tafuri Welcome message |
|
09:10 - 09:40 |
3:10-3:40 | 16:10-16:40 |
Prof. Dr. Silvio Tafuri and Dr. Antonio Di Lorenzo |
|
09:40 - 10:10 |
3:40-4:10 | 16:40-17:10 | Prof. Dr. Hidehiro Fukuyama Transdermal adjuvant for next-generation vaccines |
|
10:10 - 10:40 |
4:10-4:40 | 17:10-17:40 |
Prof. Dr. S. Louise Cosby |
|
CET |
EST |
CST Asia |
Speaker |
|
14:00-14:10 |
8:00-8:10 | 21:00-21:10 | Prof. Dr. François Meurens Welcome message |
|
14:10-14:40 |
8:10-8:40 | 21:10-21:40 |
Prof. Dr. Ger Rijkers |
|
14:40-15:10 |
8:40-9:10 | 21:40-22:10 | Prof. Dr. Ranjit Ray Modified envelope glycoproteins and the use of mRNA-LNP platform as hepatitis C virus candidate vaccine |
|
15:10-15:40 |
9:10-9:40 | 22:10-22:40 | Prof. Dr. Vishwanath Venketaraman g\Glutathione improves host immune responses against Mycobacterium tuberculosis infection |
Live Session Recordings

Keynote Speakers

Science Department, University College Roosevelt, Middelburg, The Netherlands;
St. Elisabeth Hospital, Tilburg, The Netherlands
mucosal immunology; immunoregulation (in autoimmune diseases, allergic diseases and infections); interaction between gut microbiota and the immune system; vaccination
Invited Speakers

Division of Immunology, Near InfraRed Photo-ImmunoTherapy Research Institute, Kansai Medical School, Japan

School of Medicine, Dentistry and Biomedical Sciences, Queen's University Belfast, UK

Division of Infectious Diseases, Allergy & Immunology, Saint Louis University, USA

Interdisciplinary Department of Medicine, Aldo Moro University of Bari, Italy

Department of Basic Medical Sciences, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, USA
Welcome from the Chairs
mRNA vaccines have been developed and trialled for cancers and infectious diseases. Although the success of mRNA vaccines has been achieved in the control of COVID-19, there are challenges for future development. The production of mRNA vaccines requires multiple steps including mRNA synthesis, purification, and formulation with, e.g., lipid nanoparticles. Therefore, the stability of intermediate and final products during storage and transportation is a real problem. Breakthrough infections of SARS-CoV-2 variants and the immune evasion of cancers driven by the mutation of antigens expressed from mRNA are the major challenges in development. Since mRNA vaccines have shown advantages compared to others due to the induction of humoral and cellular immune responses and adaptability in viral and tumour antigens, future vaccines should be stabler, safer, and more compatible with other regimens in combination with other options.
All vaccine-related scientists or researchers are welcome to join this event and share their findings around the following general and related themes including but not limited to:
- Vaccines to counter anti-microbial resistance;
- Vaccines to eradicate endemic disease-opportunities and challenges;
- Vaccines to prevent zoonotic transmission-targeting the animal reservoir;
- Vaccine platforms—advantages and disadvantages;
- Clinical trial design for reactive and preventive vaccination;
- Vaccine deployment in outbreak settings—lessons learned;
- Advances in vaccine formulation and adjuvantisation;
- Clinical view on routinisation of RNA vaccines;
- How do we prepare for vaccines against the as yet unknown?;
- COVID-19 Vaccine Hesitancy;
- Immune Response after Vaccination against SARS-CoV-2 in Patients with Chronic Diseases;
- T Cell Responses in SARS-CoV-2;
- SARS-CoV-2 Variant and Vaccines Development;
- Novel Technology and Vaccines Development for COVID-19;
- T Cell Immunity and HIV-1 Pathogenicity;
- Neurological Complications Following COVID-19 Infection and COVID-19 Vaccination;
- Vaccine hesitancy;
- Vaccine safety;
- Vaccine ingredients;
- Vaccine schedule;
- Vaccine objections;
- Vaccines influence on education;
- Cost-Effectiveness of vaccines;
- Vaccine transportation;
- Methods of Vaccination;
- Vaccine-related policy.
Prof. Dr. François Meurens
Dr. Fanny Renois
The Chair of the 1st International Electronic Conference on Vaccines: RNA Vaccines, Current Challenges and Future Developments
Event Chairs

Swine and Poultry Infectious Diseases Research Center (CRIPA), Faculty of Veterinary Medicine, University of Montreal, Canada
François Meurens a réalisé ses études de médecine vétérinaire en Belgique (Université Catholique de Louvain et Université de Liège) où il a obtenu son diplôme en 2000. En 2001, il a obtenu sa maîtrise en Sciences Vétérinaires (orientation virologie) et en 2004 il a terminé sa thèse de doctorat en Sciences Vétérinaires après avoir étudié la recombinaison génétique chez les alphaherpèsvirus de ruminants (Laboratoire du Professeur Etienne Thiry). Ensuite il a effectué un post-doctorat à VIDO-InterVac à l’Université de la Saskatchewan (Canada) pendant deux ans. Là-bas il s’est intéressé à l’immunologie des muqueuses dans les espèces porcine et ovine (laboratoire du Pr Volker Gerdts). Fin 2006 il a intégré l’INRA de Tours (unité ISP) en qualité de chargé de recherche. Sur place il a animé une équipe spécialisée dans l’étude des relations hôte/pathogène dans le modèle porcin. En 2012 il a ensuite accepté un poste de Scientique/Professeur adjoint à VIDO-InterVac (Canada) où il a animé une équipe sur des thématiques très similaires à celles développées en France. Une attention particulière a été portée à l’intérêt du porc en tant qu’espèce modèle pour la recherche biomédicale humaine. Auteurs de nombreuses publications dans des journaux internationaux à comité de lecture et exerçant des activités d’experts pour le secteur privé et dans les comités éditoriaux des revues Veterinary Immunology and Immunopathology et Frontiers in Veterinary Medicine, le Dr Meurens s’est orienté en 2015 vers une carrière plus académique alliant l’enseignement à la recherche scientifique à Oniris. Depuis lors il exerce son activité d’enseignement en microbiologie/immunologie et son activité de recherche au sein de l’UMR BioEpAR.
francois.meurens@umontreal.ca

Short biography Fanny Renois (PhD, HDR) began her career as an immuno-virologist at the Faculty of Medicine and the University Hospital of Reims (France). Her first project focused on Picornaviridae with respiratory tropism in humans, which were awarded the RICAI Prize in 2012. Then, Fanny Renois developed her research on the elucidation of the role of Coxsackieviruses and their mechanism of action in the development of dilated cardiomyopathies and sudden death in young adults. Since 2016, Fanny Renois has been an associate professor of public health at the National Veterinary School of Nantes (France). Currently, she is developing research to understand the molecular aspects of the innate immune response developed against multiple respiratory viruses in the porcine species.
fanny.renois@umontreal.ca
Session Chairs

Dr. Silvio Tafuri
Interdisciplinary Department of Medicine, Aldo Moro University of Bari, Italy
Silvio Tafuri was born in Brindisi (Italy) on 1980, 17 August. He graduated in Medicine and Surgery at Bari School of Medicine in 2005; In 2008, he achieved post-degree in Public Health and since 2013 he is Ph.D. in Hygiene, Public Health and Food Safety. He is a consultant of the Apulian Observatory for Epidemiology in the field of vaccination strategies, surveillance of infectious diseases and of adverse events following immunization. From 2014 to March 2020, he has been Assistant Professor of Public Health at the Bari School of Medicine; since March 2020 to April 2021, he was Associate Professor of Public Health. From April 2021, he is Full Professor at the University of Bari. He works as consultant in the Public Health Unit of the Policlinico Bari General Hospital, in which he is charged of the management of vaccination activities; since March 2020, he is Chief of the Control Room Unit and of the Infection Control Program Team. He is author of 280 articles published in international scientific journals (IF >700, h-index 27); the major part of scientific paper is in the field of vaccinology and of the prevention of infectious diseases.

Prof. Dr. Martin J. D'Souza
Department of Pharmaceutical Sciences, Mercer University, USA
Dr. D’Souza’s Nanotechnology laboratory mainly focuses on the design and delivery of both Buccal vaccines using oral dissolving film (ODF) and Transdermal vaccines using microneedles for infectious diseases and cancer. Currently Dr. D’Souza’s lab is focusing vaccines for Infectious diseases such as SARS CoV-2, Universal Influenza, RSV, Zika, and Gonorrhea – to name a few. Additionally, work is underway on therapeutic vaccines for cancers such as Melanoma, Ovarian, Prostate ad Breast. Dr. D’Souza has graduated over 60 Ph.D. students and has published over 150 manuscripts. He has been the recipient of several research grants from the National Institutes of Health (NIH), the American Diabetes Association, the Georgia Cancer Coalition, the Georgia Research, Alliance. He serves as an Executive Editor, and is on the Editorial Boards of over 10 Scientific Journals. He has several patents issued in the area of Nanotechnology. Currently, he has several active NIH funded research grants

Dr. Siddappa N. Byrareddy
1. Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, USA 2. Department of Genetics, Cell Biology and Anatomy, University of Nebraska Medical Center, USA 3. Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, USA
Dr. Siddappa Byrareddy, Ph.D., is currently a Professor & Vice Chair of Research in the department of Pharmacology & Experimental Neuroscience at the University of Nebraska Medical Center, Omaha, NE, USA. Dr. Byrareddy's current research interests include understanding and developing therapeutics for emerging and reemerging infectious diseases focusing on HIV, Zika, and SARS-CoV-2. His lab emphasizes understanding viral reservoirs (including T cells and myeloid cells), inflammation, and developing antivirals, vaccines and animal models for infectious diseases. Dr. Byrareddy's research is supported by multiple NIH awards (R01s, U01, R21), he serves on numerous review panels and has published more than 170 refereed journal articles

Dr. Daniel Dory
ANSES - FRENCH AGENCY FOR FOOD, ENVIRONMENTAL AND OCCUPATIONAL HEALTH & SAFETY Laboratory of Ploufragan/Plouzane/Niort Viral Genetics and Biosafety, France
Daniel DORY performed a first post-doc at the University Hospital of Basel (Switzerland) where he studied cellular receptors involved in the innate immune responses against bacteria. Thereafter he moved to ANSES (France) where he invistigated different strategies to improve DNA vaccination efficacy in pigs against infectious diseases (adjuvants, plasmid backbones, electroporation, heterologous prime-boost regimen …). He studied also the biodistribution of plasmid DNA upon intramuscular injection in pigs. DNA vaccination was also applied in chickens and in fish.

Dr. Angelos Hatzakis
Department of Hygiene, Epidemiology and Medical Statistics, National and Kapodistrian University of Athens Medical School, Greece; Hellenic Scientific Society for the Study of AIDS and Sexually Transmitted Diseases, Greece; Hepatitis B and C Public Policy Association, Luxembourg
Short biography Angelos Hatzakis MD, PhD Professor of Epidemiology and Preventive Medicine Department of Hygiene, Epidemiology and Medical Statistics, National and Kapodistrian University of Athens, Medical School Hellenic Scientific Society for the Study of AIDS, Sexually Transmitted and Emerging Diseases Former President of the Hellenic Center for Disease Control & Prevention. Founder of the National Retrovirus Reference Center, Athens, Greece. Founder and Co-Chair of “Hepatitis B & C Public Policy Association”, Luxembourg. Participation in many Executive Committees of public and private sector. His research interests cover epidemiology, virology, preventive medicine, Public Health and infectious diseases, especially HIV/AIDS, hepatitis, COVID-19, and pandemic viruses, harm reduction and mortality patterns of PWID’S. Author or co-author of more than 350 scientific papers published in Journals like Lancet, Lancet HIV, Lancet Gastroenterology and Hepatology, JAMA, British Medical Journal, Annals of Internal Medicine, PNAS, Hepatology, Gastroenterology, AIDS, Journal of AIDS, Journal of Infectious Diseases, Clinical Infectious Diseases, Blood, Cancer Research, Pediatrics etc. with more than 21.000 citations.
Event Committees

Department of Public Health Policy, School of Public Health, University of West Attica, Greece
Dimitris Zavras is Assistant Professor of Health Services Research and Health Economics at the Department of Public Health Policy at the University of West Attica. Along his academic, educational and research responsibilities he serves as Director of the Division of Health Systems and Policy. He is also Faculty Member of the Hellenic Open University. Dimitris was born in Athens in 1968 and studied Physics at the National & Kapodistrian University of Athens. His postgraduate studies include an M.Sc. in Applied Physics from the University of Massachusetts; M.Sc. in Statistics from Athens University of Economics and Business; M.Sc. in Healthcare Management from the National School of Public Health; and a Doctorate (Ph.D.) in Health Services Research and Health Economics from the University of Thessaly (Department of Economics). Dimitris has taught in postgraduate programs of the University of West Attica, the University of Peloponnese, the Neapolis University Pafos (Cyprus), and the National School of Public Health and in undergraduate programs of the University of West Attica, the University of Peloponnese and the Athens University of Applied Sciences. Dimitris Zavras’ research interests focus on healthcare access and economics, the utilization of healthcare services, healthcare provider choice, and unmet healthcare needs. His most recent research work and published articles deal with economic issues surrounding the COVID-19 pandemic. He is a member of the International Health Economics Association, the Hellenic Scientific Society of Health Economics and Health Policy and the Hellenic Society of Public Health. He is also a member of the Hellenic Society for Healthcare Services Management.
Healthcare access; utilization of healthcare services; healthcare provider choice; unmet healthcare needs; access to the COVID-19 vaccine; economic impact of the COVID-19 pandemic; vaccination

I received a Ph.D. in microbiology from Zhejiang University, China in 2015, where I studied oxidative stress adaption and flagellar regulation of Shewanella oneidensis. I then continued my training as a postdoctoral scholar at the University of Chicago’s Department of Microbiology since 2015 with focusing on pathogenesis of Staphylococcus aureus and vaccine development. until 2019, and currently is a research professional at the University of Chicago’s Howard Taylor Ricketts Laboratory.
Staphylococcus aurues, phathogenesis, vaccine, antibody engineering

12735 Twinbrook pkwy 3W05 Laboratory of Malaria and Vector Research National Institute of Allergy and Infectious Disease National Institutes of Health Rockville, USA
Gagandeep Singh Saggu completed his Ph.D. in Molecular parasitology from the Department of Biological Sciences, BITS, Pilani, Pilani Campus. He was awarded a Junior and Senior Research Fellowship (Dec 2010) from the Council of Scientific and Industrial Research and University Grant Commission, Govt. of India to pursue Ph.D. During his Ph.D. He has published research articles in journals of international repute and has presented his research work at national and international conferences and workshops. He joined the National Institute of Allergy and Infectious Diseases, NIH as a Postdoctoral Research Fellow. In NIAID he continued his research work in the field of parasitology and with the help of Patch-clamp identified a novel ion channel/transporter on the digestive vacuole membrane of the malaria parasite. He was successful to study the various properties and selectivity of these channels. He is in the process of molecular characterization of these channels which will provide foundational insights into vacuolar biology, clarify the resistance mechanisms for several antimalarial drugs, and guide the development of new therapies for malaria. For his current research, he received the ASTMH research excellence award in the field of molecular biology and the Fellowship Award for Research Excellence (FARE 2019) from NIH.
Malaria, Plasmodium, Electrophysiology, CRISPR, Parasite transfection, Advance microscopy

Greg Simons was born in New Zealand in 1969 and graduating with a PhD from the University of Canterbury in 2004, and promoted to Associate Professor by the Department of Government at Uppsala University in 2015. Greg Simons is currently a researcher at the Institute for Russian and Eurasian Studies (IRES) at Uppsala University, and a lecturer at the Department of Communication Science at Turiba University in Riga, Latvia.
crisis management and crisis communications (including public health issues), changing political dynamics and relationships, mass media, public diplomacy, political marketing, media and armed conflict, and the Russian Orthodox Church

1. Laboratory of Computing, Medical Informatics and Biomedical - Imaging Technologies, School of Medicine. Aristotle University of Thessaloniki
2. International Hellenic University
34 publications in international scientific journals, more than 55 announcements to international scientific conferences; 2011 School of Biology, AUTH 2013 MSc in Applied Genetics and Biotechnology, School of Biology, AUTH 2018 PhD in Human Genetics-Immunogenetics , School of Medicine, AUTH 2019 Post-doc researcher in School of Medicine, AUTH 2020 MSc in Medical Informatics, School of Medicine, AUTH
Genetics/Immunogenetics, Next Generation Sequencing, Flow cytometry

Dr. Zanin received his PhD in Microbiology from Monash University in Melbourne, Australia and underwent postdoctoral training in influenza virology at St. Jude Children’s Research Hospital in Memphis, TN, USA with Dr. Robert Webster and Dr. Richard Webby. He subsequently established a research group at the State Key Laboratory of Respiratory Diseases in Guangzhou, China to study the emergence and transmission of zoonotic respiratory viruses.
Dr. Zanin’s research interests are the host and viral factors underlying transmission of respiratory viruses and interventions against viral respiratory diseases.

University of Cologne, Faculty of Medicine, Department I of Internal Medicine, Center for Integrated Oncology Aachen Bonn Cologne Duesseldorf (CIO ABCD) and Excellence Center for Medical Mycology (ECMM) and Institute of Translational Research, Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD) , Germany
Dr. Jon Salmanton-García, PhD in Health Sciences by the University of Cologne (Cologne, Germany) is a postdoctoral researcher and project manager in the group of Prof. Dr. Oliver A. Cornely, Department I of Internal Medicine, University Hospital Cologne (Cologne, Germany). He did his PhD thesis on emerging invasive fungal infections, namely mucormycosis, and extremely rare invasive fungal infections within the FungiScope – Global Registry of Emerging Fungal Infections team (www.fungiscope.net). Currently, he is a member of the Coordination Office of the VACCELERATE Consortium, a pan European clinical research network for the coordination and conduction of COVID-19 vaccine trials (www.vaccelerate.eu), managing the EUVAP – European Vaccine Trial Accelerator Platform (www.euvap.eu), so as the VACCELERATE Volunteer Registry (www.vaccelerate.eu/volunteer-registry). In parallel, he also coordinates the EPICOVIDEHA survey – Epidemiology of COVID-19 infection in patients with haematological malignancies: A European Haematology Association Survey (www.ehaweb.org/covid-19/epicovideha-survey/). Additionally, he is in charge of the research series “The current state of laboratory mycology in…”, an online survey where institutions from different geographical regions are being asked about their diagnostic and treatment capacities for invasive fungal infections.
He has a special interest in registries and questionnaires, database management, invasive fungal infections and infectious diseases in general.

Key Laboratory of Veterinary Biological Engineering and Technology, Ministry of Agriculture, Jiangsu Co-innovation Center for Prevention and Control of Important Animal Infectious Diseases and Zoonoses, Yangzhou University, China
Li Yin: Doctor of Preventive Veterinary Medicine, Researcher. He is a master’s degree supervisor at Nanjing Agricultural University and Jiangsu University, and a postdoctoral supervisor at the postdoctoral station of Jiangsu Academy of Agricultural Sciences. Main research content and direction: The mutation, cross-host transmission mechanism, and prevention and control of avian influenza virus subtype H9; the influence mechanism of structural and non-structural proteins of waterfowl tembusu virus on virulence, pathogenicity and immunogenicity of the virus and its prevention and control; the epidemiological investigation, pathogenic mechanisms, and prevention and control of the goose astrovirus. Waterfowl Disease Prevention and Control Innovation Team, Institute of Veterinary Research, Jiangsu Academy of Agricultural Science.

Coxiella Pathogenesis Section, Laboratory of Bacteriology, Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health, USA
Dr. Long graduated summa cum laude from Gardner-Webb University with a B.S. in 2011. She received her Ph.D. in immunology and microbial pathogenesis from West Virginia University in 2016. Here, she studied the role of regulatory T cells and microRNAs in chemical allergy at the National Institute for Occupational Safety and Health (CDC). After earning her doctorate, Dr. Long moved to Hamilton, Montana, to join Dr. Robert Heinzen's group at the National Institutes of Health to work as an Intramural Research Training Award postdoctoral fellow. During this time, Dr. Long worked in the biosafety level 3 laboratory researching the causative agent of Q fever, Coxiella burnetii. She investigated both bacterial and host factors required for virulence and refined guinea pig models for infection, vaccination, and post-vaccination hypersensitivity. In 2019, Dr. Long received an Independent Research Scholar Award from NIH, allowing her to form an autonomous research group to continue her work on Coxiella burnetii.
Coxiella burnetii, bacterial vaccines, bacterial genetics, adaptive immune responses, host-pathogen interactions, dermal allergy, post-vaccination hypersensitivity, Q fever, lipopolysaccharide
Instructions for Authors
Note: Institutional email address is requested especially for the corresponding author. Please submit the abstract along with an institutional email address.
1. Scholars interested in participating in the conference can submit their abstract (about 200–300 words) online on this website until
11th July 2023 15th September 2023 . The Conference Committee will notify regarding acceptance of the abstract by 25th July 2023 27th September 2023.
2. In case of acceptance, authors will be asked to submit their manuscript (short proceedings paper, 3–6 pages), a poster, a slide presentation (in PDF), or a short video presentation (max. 3–5minutes) before 13th September 2023 20th October 2023. Authors will receive notification about the acceptance of their paper by 30th September 2023 20th November 2023.
3. The manuscripts and presentations will be available on Sciforum.net for discussion and rating during the time of the conference, 1–15 December 2023.
Note: Publication of proceedings paper is free of charge. Before publication, Medical Sciences Forum journal will check the plagiarism issue again. Submissions with a lack of novelty will not be published in the journal.
The submission to the Special Issue is independent of the conference proceedings and will follow the usual process of the journal, including peer review, APC, etc. All participants of IECV 2023 are welcome to submit an extended full paper to the conference Special Issue "Selected Papers from the 1st International Electronic Conference on Vaccines: RNA Vaccines, Current Challenges and Future Developments (IECV2023)" of the journal Vaccines, with a 20% discount on the article processing charge.
Proceedings Papers
Manuscripts for the proceedings issue must be structured as follows:
- Title
- Full author names
- Affiliations (including full postal address) and authors’ e-mail addresses
- Abstract
- Keywords
- Introduction
- Methods
- Results and Discussion
- Conclusions
- (Acknowledgments)
- References
Video Presentations
Posters
2)The minimum size for images is 148 mm × 210 mm (horizontal × vertical) at 300 dpi.
3)The content of the poster should be a comprehensive presentation of your accepted submission.
4) No copyright issues with any elements in the poster.
Potential Conflicts of Interest
Copyright
List of accepted submissions (6)
| Id | Title | Authors | Presentation Video | Poster PDF | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sciforum-080812 | Perception, attitude and intention towards COVID-19 vaccination | , , , | N/A |
|
Show Abstract |
||||||||||||||||||||||||||||||||||||
|
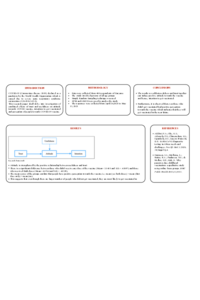
To overcome the situation due to pandemic (COVID-19), vaccination became essential. So, it was important to understand the overall perception, attitude and intention of respondents towards vaccination. This study aimed to investigate the combined effect of usefulness and trust on attitude towards COVID-19 vaccination and to understand the perception of vaccinated and unvaccinated people towards vaccination. Self administered questionnaire was used to collect the data. In the descriptive research design, Structural Equation Modeling was used to test the combined effect of usefulness and trust on attitude towards COVID-19 vaccination and one-way ANOVA was used to test the difference in perception of vaccinated and unvaccinated people. Simple random sampling was used in this study. The questionnaire based data were collected from 400 respondents of Haryana from April 24, 2021 to May 13, 2021. As per results, more than 70% were not vaccinated, around 16% received their first dose of vaccine and less than 15% received both doses of vaccine. Usefulness and trust had an impact on the attitude towards vaccination. There was significant difference between those who didn’t receive any dose of the vaccine i.e either Covishield (viral vector vaccine) or Covaxin (inactivated viral vaccine) and those who received both doses of the vaccine. The results reveal that attitude is strengthened by positive relation between trust and usefulness. Even though there were large number of people who were not vaccinated at the time of the survey, these people had positive perception towards the vaccine. So, they were most likely to get vaccinated in the future. It was also found that vaccine history of the respondents played an important role in future vaccination. Awareness programmes become important as people need to be well informed about the benefits of vaccination. |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-080541 | Impact of COVID-19 on Influenza Virus Vaccination Coverage | , , , | N/A | N/A |
Show Abstract |
||||||||||||||||||||||||||||||||||||
|
INTRODUCTION: Influenza vaccination is pivotal in alleviating healthcare burdens and possibly curbing COVID-19 infections due to symptom overlap. Despite previous suboptimal vaccination rates, the exceptional circumstances of the pandemic may have impacted Influenza vaccine coverage in 2020 and 2021. This study examines Influenza vaccination adherence from 2019 to 2021 at a private vaccination clinic in Belo Horizonte, Brazil, while considering demographic variables. METHODS: Quantitative analytical study of a cross-sectional nature. Participants remained anonymous, identified solely by registration numbers to prevent data duplication. The sample comprised 36,478 individuals who sought the quadrivalent influenza vaccine at a private vaccination clinic in Belo Horizonte, Brazil, between January and December 2019 to 2021. Data, including vaccination date (month and year), age, and gender of participants, were collected and analyzed directly from the company's database, and correlations with the epidemiological variation of COVID-19 in the country during the period were established. RESULTS AND DISCUSSION: Over the study period, vaccination adherence showed a steady 20.69% increase, with a noteworthy peak in vaccinations occurring in April (41.9%). The 40 to 59-year-old age group (33.4%) emerged as the most prominent, comprising a substantial portion of the workforce, putting them at considerable risk of severe COVID-19 cases. Notably, our analysis unveiled a valuable correlation between influenza vaccination and COVID-19 case notifications, indicating heightened demand for immunization during the pandemic. These findings significantly contribute to our comprehension of vaccination patterns during health crises. CONCLUSION: This study demonstrates a notable rise in Influenza vaccination coverage during the COVID-19 pandemic. Socio-educational measures and government vaccination incentives seem to have played a significant role in this increase. Nonetheless, the substantial surge observed between 2019 and 2020 is likely linked to the population's heightened COVID-19-related apprehensions. |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-074340 | Challenges faced by states and the WHO in regulating efficiently the use of mRNA vaccines | N/A | N/A |
Show Abstract |
|||||||||||||||||||||||||||||||||||||
|
According to the World Health Organization (WHO), there is no formal regulatory guidance specifically for mRNA-based vaccines[1]. However, WHO provides information and regulatory considerations regarding key aspects of the manufacture and quality control, and nonclinical and clinical evaluation, of preventive mRNA vaccines against infectious disease for human use[2]. The global research and development of mRNA vaccines have been prodigious over the past decade, and the work in this field has been stimulated by the urgent need for rapid development of vaccines in response to an emergent disease such as the current COVID-19 pandemic[3]. In the EU no regulatory guidelines presently exist that specifically address mRNA-based vaccines. The existing regulatory framework, however, clearly defines that mRNA-based vaccines in most cases have to be centrally approved[4]. In the UK, both mRNA vaccines were granted temporary regulatory authorization under Regulation 174 of the Human Medicine Regulations 2012[5]. The potential of mRNA vaccine as a technology to rapidly respond to public health emergencies of infectious diseases, in addition to application for prophylactic vaccines for additional infectious diseases, have underscored the need for international regulatory convergence for RNA vaccines[6]. The challenges faced by states in the use of mRNA vaccines include not only regulatory gaps and but also technical issues such as the need for cold storage and transportation. [1] Evaluation of the quality, safety and efficacy of RNA-based prophylactic vaccines for infectious diseases: regulatory considerations https://www.who.int/docs/default-source/biologicals/ecbs/reg-considerations-on-rna-vaccines_1st-draft_pc_tz_22122020.pdf?sfvrsn=c13e1e20_3. [2] Messenger RNA vaccines - World Health Organization (WHO). https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/mrna-vaccines. [3] Development of mRNA Vaccines: Scientific and Regulatory Issues. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7910833/. [4] The European Regulatory Environment of RNA-Based Vaccines. https://pubmed.ncbi.nlm.nih.gov/27987152/. [5] Spotlight On MRNA – Regulation of MRNA Vaccines and Therapies - Life .... https://www.mondaq.com/uk/life-sciences-biotechnology-nanotechnology/1133714/spotlight-on-mrna-regulation-of-mrna-vaccines-and-therapies. [6] See: Messenger RNA vaccines - World Health Organization (WHO). https://www.who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/mrna-vaccines.; Evaluation of the quality, safety and efficacy of RNA-based .... https://www.who.int/docs/default-source/biologicals/ecbs/reg-considerations-on-rna-vaccines_1st-draft_pc_tz_22122020.pdf?sfvrsn=c13e1e20_3.; Development of mRNA Vaccines: Scientific and Regulatory Issues. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7910833/. |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-072818 | Factors driving the attitudes and hesitancy of Albanian parents toward Covid-19 vaccination of children. | , | N/A | N/A |
Show Abstract |
||||||||||||||||||||||||||||||||||||
|
Background The “3Cs” model of hesitancy claims that vaccine hesitancy is influenced by three factors such as complacency, convenience, and confidence which drive the acceptance or refusal of vaccines. Vaccine hesitancy is context-, time-, place- and vaccine-specific. This study aims to analyze different factors influencing perceptions and attitudes of Albanian parents toward covid-19 vaccination of children. The Anti-Covid-19 vaccine remains unapproved for children younger than 12 in Albania. Methods A validated questionnaire composed of 33 elements was used for the purpose of this study. The study was conducted on a sample of parents/caregivers of children aged 0 months to 18 years. There first section of the questionnaire is composed of demographic questions. The other sections aimed to collect data about economic level, political status of the country where they live, policies toward vaccination, parent’s perceptions and beliefs about covid-19 vaccines in children and access to immunization settings. The survey tool was composed of questions aimed to measure the complacency, convenience and confidence of the respondents toward Covid-2. The data collected from the questionnaire were first coded and then studied using statistical software STATA13 and SPSS21. The data analysis was done based on 3 domains.
For each of these sub-divisions (sections), descriptive statistics (frequencies and percentages) and assessment of the association of different variables (socio-demographic; experience with COVID; parental perceptions on COVID) with the basic variable which is "perception of safety of the ANTI COVID 19 vaccine in children" through Pearson's chi-square test was conducted. Then, using the multinomial regression method, the factors that influence the concrete administration of vaccination among children were analyzed. For this purpose, the following are considered as independent variables: age; the country where Albanians live; schooling; vaccination obligation in the respective state; the child's experience with COVID-19; the perceived safety of the COVID-19 vaccine (categorized in: do not agree at all; partially agree; completely agree). Data were analyzed using the SPSS statistic program and R-project. Results A total of 600 parents/caregivers responded to the questionnaire. The parents/caregivers that responded to the questionnaire were categorized in 3 groups: Albanian parents living in Albania, Albanian parents living in diaspore, Albanian parents living in Kosovo. The three groups were confronted between them in terms of perceptions and attitudes toward vaccination of children aged 0-18 years old with the SARS Cov-19 vaccine. 94.5% of the respondents were represented by mother. 52% of the respondents had a University degree and 33.1% of them had a post-graduate degree such as Doctoral studies, Master or Specialization diploma. 58% of the respondents declared to have a middle income. The age of the children in 39% of the cases was 0-2 years old, 16% of the respondents were parents of children aged 12-18 years old. 76% of the respondents declared that they would not vaccinate their child with the Covid-19 vaccine. 50% of them did not consider important the administration of covid-19 vaccine to their children. From the regression analysis the following results were obtained
Conclusion From the results of this study several factors seem to influence the perceptions of parents toward Covid-19 vaccination of their children. The negative perceptions toward vaccination of their child were linked to mild form of the disease passed by their children and fear of adverse events. While access to immunization settings and economic level seemed to not influence the attitudes of parents toward vaccination. The perceived safety of the Anti-Covid-19 vaccine had a real impact on the implementation of vaccination in children: the positive perception of the safety of the Anti-Covid-19 vaccine was related to decreased refusal to administer the vaccination to children. The negative perceptions of parents toward covid-19 vaccine seems to influence their attitude toward other childhood vaccinations, delaying the immunization time of their child which could by itself result in an increase of incidence of vaccine preventable diseases. |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-080932 | Community Level Correlates of COVID-19 Booster Vaccine Hesitancy in the United States: A Cross-Sectional Analysis |
,
,
Crysty-Ann Olaco ,
,
,
|
N/A |

|
Show Abstract |
||||||||||||||||||||||||||||||||||||
|
Introduction The COVID-19 vaccine was the first mRNA vaccine to receive full FDA approval. It has been shown that the novelty of mRNA vaccines significantly increases vaccine hesitancy, possibly due to the spread of misinformation on this technology. Additionally, previous research has indicated that individual-level socio-demographic factors contribute to vaccine hesitancy. However, it is unknown how community-level factors affect COVID-19 booster dose hesitancy. The current study aims to fill this knowledge gap by comparing data from a nation-wide survey on COVID-19 vaccine hesitancy with a community-level indicator i.e. Distressed Communities Index (DCI). Methods This cross-sectional study utilized a 48-item, psychometrically valid and reliable tool to measure attitudes toward vaccinations, vaccine literacy, COVID-19 vaccine confidence index, and trust in July 2021. A total of 2,138 survey participants residing in the United States were divided into quintiles of varying community distress levels (ranging from 1=prosperous to 5=distressed) based on their zip codes using the DCI. Data were analyzed through Chi-square, one-way ANOVA, and post-hoc analysis with Tukey’s test. Results A significantly larger proportion of participants from the distressed communities had lower trust as opposed to their prosperous counterparts (26.6% vs. 37.6%, p<0.001). On the contrary, participants from the prosperous communities had the significantly higher mean scores of the vaccine confidence index as opposed to those who were living in distressed communities (2.22±1.13 vs. 1.70±1.01, p<0.001). Conclusion These findings affirm the importance of developing community-level interventions in these more vulnerable groups to promote trust in COVID-19 vaccinations, thereby increasing COVID-19 booster dose uptake. From these results, future studies can examine the efficacy of various community-level interventions in promoting vaccine confidence in communities with higher rates of vaccine hesitancy. |
|||||||||||||||||||||||||||||||||||||||||
Event Awards

To acknowledge the support of the conference esteemed authors and recognize their outstanding scientific accomplishments, we are pleased to announce that the conference will provide one Best Paper Award and one Best Presentation Award.
Best Paper Award Certificate
Author: Rabaï Bouderhem
ID: sciforum-074340
Title: Challenges faced by states and the WHO in regulating efficiently the use of mRNA vaccines
Link: https://sciforum.net/paper/view/16530
Best Presentation Award Certificate
Authors: Jeffrey Martin; Henry Krasner; Crysty-Ann Olaco; Nicolette Harmon;
Kavita Batra; Dale Netski;
ID: sciforum-080932
Title: "Community Level Correlates of COVID-19 Booster Vaccine Hesitancy in the United States: A Cross-Sectional Analysis"
Link: https://sciforum.net/paper/view/16576
The Awards
Number of Awards Available: 1
Vaccines would like to grant an award for the best paper as elected by the conference committee.
Terms and Conditions:
- Proceedings paper (3–6 pages) must be submitted to IECV 2023;
- Originality/Novelty of the paper;
- Significance of Content;
- Scientific Soundness;
- Interest to the readers;
- English language and style.
Number of Awards Available: 1
Vaccines would like to grant an award for the best presentation at the conference as determined by a jury. Poster/Slides/Video Presentations will be considered for this award.
Presentations should have the following information:
- Title (with authors and affiliations)
- Introduction/Objectives/Aims
- Methods
- Results
- Conclusion
- Acknowledgments
- Contact information
Conference Secretariat
Ms. Cynthia Wang
Ms. Alethea Liu
For inquiries regarding submissions and sponsorship opportunities, please feel free to contact us.
E-Mail: iecv@mdpi.com
S1. COVID-19 Vaccines and Vaccination
Session Chairs
Dr. Siddappa N. Byrareddy, 1. Department of Pharmacology and Experimental Neuroscience, University of Nebraska Medical Center, USA 2. Department of Genetics, Cell Biology and Anatomy, University of Nebraska Medical Center, USA 3. Department of Biochemistry and Molecular Biology, Un
Dr. Angelos Hatzakis, Department of Hygiene, Epidemiology and Medical Statistics, National and Kapodistrian University of Athens Medical School, Greece; Hellenic Scientific Society for the Study of AIDS and Sexually Transmitted Diseases, Greece; Hepatitis B and C Public Polic
S2. Vaccines against Infectious Diseases
Session Chairs
Prof. Dr. Martin J. D'Souza, Department of Pharmaceutical Sciences, Mercer University, USA
Dr. Daniel Dory, ANSES - FRENCH AGENCY FOR FOOD, ENVIRONMENTAL AND OCCUPATIONAL HEALTH & SAFETY Laboratory of Ploufragan/Plouzane/Niort Viral Genetics and Biosafety, France
Show all accepted abstracts (2) Hide accepted abstracts (2)
List of Accepted Abstracts (2) Toggle list
S3. Vaccines and Society
Session Chair
Dr. Silvio Tafuri, Interdisciplinary Department of Medicine, Aldo Moro University of Bari, Italy

































