The 2nd International Electronic Conference on Antibiotics
Drugs for Superbugs: Antibiotic Discovery, Modes of Action And Mechanisms of Resistance
Part of the International Electronic Conference on Antibiotics series
15–30 June 2022
antibiotics use, Antimicrobial Resistance, Mechanisms of antibiotic action
- Go to the Sessions
-
- S1. Antimicrobial Discovery, Development & Optimization
- S2. Mechanisms of Action & Resistance
- S3. Epidemiology & Multidrug Resistance
- S4. Repurposing & Antimicrobial Adjuvants
- S5. Antimicrobials & Interfaces
- S6. Clinical Studies
- S7. Initiatives and Policies on Antimicrobial Development and Resistance
- P. Poster
- Event Details
-
- Welcome from the Chairs
- Sessions
- Event Chairs
- Event Speakers
- List of Keynotes & Videos
- Live Sessions - Registration and Program
- Live Session Recordings
- List of Accepted Submissions
- Poster Gallery
- Event Calls
- Instructions for Authors
- Event Awards
- Conference Secretary
- Sponsors and Partners
- Events in series ECA
ECA2022 is closed. Thank you for your participation.
The recordings of ECA2022 live sessions are available at:
https://eca2022.sciforum.net/#recordings
The accepted proceedings papers will probably be published as one dedicated volume in journal Medical Sciences Forum (ISSN 2673-9992) after the conference.
All participants of ECA2022 are also welcome to submit the extended work to the Antibiotics (ISSN 2079-6382, IF 5.222) Special Issue "Drugs for Superbugs: Antibiotic Discovery, Modes of Action and Mechanisms of Resistance".
The ECA2022 two award winners have been announced at https://eca2022.sciforum.net/#awards.
Welcome from the Chairs

Antibiotics have proven to be powerful drugs for the control of infectious diseases and remain one of the most significant discoveries in modern medicine. The development of antimicrobial resistance is the consequence of decades of constant selective pressure via the underuse, overuse and misuse of therapeutic molecules, which highly benefit the extraordinary genetic capacities of microorganisms. The remarkable plasticity of bacterial genomes in addition to the capacity to acquire and transmit genetic determinants of resistance are conserved evolution strategies and have exacerbated the worldwide resistance problem.
The resistance of pathogenic bacteria to individual antibiotics is a great problem by itself. However, the emergence of multidrug-resistant strains represents an increasing complication in the treatment of bacterial infections. The worldwide spread of multidrug-resistant microorganisms is increasingly drawing the attention of global surveillance authorities, being undoubtedly rated as a major threat in the 21st century. Each year, circa 13 million deaths in the world are attributed to the emergence of new infectious diseases or to new resistance determinants. A harsh prediction for the close future is that the treatment of certain types of severe infections will come to a therapeutic dead end. This situation raises the fear of a "post-antibiotic era" of resistant superbugs, a prediction that is made more real each day. This is particularly critical for Gram-negative bacteria.The quest for new antimicrobials to overcome resistance problems has long been a top research priority. However, scientific difficulties and low financial returns have led to the withdrawal of many pharmaceutical companies from antibiotic R&D. On the other hand, academia is still highly prolific in R&D on antibiotic discovery from the most diverse sources, their modes of action and mechanisms of resistance.
The role of antibiotics cannot be overestimated. Actions for improving their clinical performance and/or developing more sophisticated systems to effectively treat infectious diseases are needed. Nowadays, we know that bacteria will resist and persist all kinds of antibiotics. This is easy to understand if we consider that bacteria, during the course of their evolution, faced situations even more difficult than antibiotic exposure. Additionally, this becomes worst when bacteria act as a community, as a biofilm.
It is our aim that this event can provide an opportunity to representatives of academia, industry and health services to discuss and advance our current knowledge on how to control microorganisms.
We welcome discussions of the following topics at the event:
- Drug discovery and development—from natural sources to (bio)synthesis;
- Drug repurposing for antimicrobial therapy;
- Antibiotic recycling and resistance modifiers;
- Virtual screening;
- Susceptibility testing;
- Mechanisms of action;
- Anti-virulence;
- Pharmacological parameters, including in vitro or in vivo modelling studies;
- Novel delivery systems for antimicrobials;
- Mechanisms of resistance—from natural to acquired;
- Cross-resistance;
- Ohmics in antibiotic action and resistance;
- Epidemiology and the emergence of antimicrobial resistant pathogens;
- Clinical trials and clinical cases of antimicrobial resistance;
- Antibiotics and interfaces.
Sessions
S2. Mechanisms of Action & Resistance
S3. Epidemiology & Multidrug Resistance
S4. Repurposing & Antimicrobial Adjuvants
S5. Antimicrobials & Interfaces
S6. Clinical Studies
S7. Initiatives and Policies on Antimicrobial Development and Resistance
P. Poster
Event Chairs

Department of Chemical Engineering, Faculty of Engineering, University of Porto, Portugal
Manuel Simões has a PhD in Chemical and Biological Engineering and is currently Associate Professor with Habilitation at the Faculty of Engineering of the University of Porto, where he is Pro-Director. He has more than 180 papers published in journals indexed in JCR (h-index = 48), 4 books (1 as author and 3 as editor) and more than 40 chapters in books. His research focusses on biofilms and antimicrobial action and resistance. He has been highlighted as a highly cited author in 2020 and 2021 by Clarivate Analytics. SCOPUS ID: 55608338000; Orcid ID: 0000-0002-3355-4398.

CNRS, Aix-Marseille University, Centrale Marseille, iSm2, Marseille, France
Dr. Marc Maresca is currently a researcher at Aix-Marseille Université. He received his Ph.D. in Biochemistry from the Université Paul Cézanne (France, 2003) working on food contaminants named mycotoxins. In 2003, he moved to England to work on enteropathogenic E coli in Brendan Keny’s lab. Then, he moved back to France to continue his work on mycotoxins and their effects on human health. He is currently working at Aix-Marseille Université and his research aims to identify and develop new molecules—natural, synthetic, or bio-inspired—with antimicrobial properties. He focuses on antimicrobial peptides and their mimics as well as on plant derivatives; in addition to their antimicrobial effects, they may possess additional activities such as anti-inflammatory and anti-tumorigenic effects.
Event Committee

Molecular Horizons and School of Chemistry and Molecular Bioscience, University of Wollongong, NSW 2522, Australia
Prof. Dr. Nick Dixon holds a PhD in Biochemistry from the University of Queensland (1978). He was a Research Fellow with Prof Alan Sargeson (Research School of Chemistry, ANU) before being awarded C.J. Martin and Fulbright Fellowships to study with Nobel laureate Prof Arthur Kornberg at Stanford University, U.S.A. He returned to the John Curtin School of Medical Research at ANU as a Queen Elizabeth II Fellow in 1983, and was appointed as Fellow in Biological Chemistry at the Research School of Chemistry, ANU in 1986. He was subsequently promoted to Senior Fellow and Professor, before leaving ANU in 2006 to take up his present position as Professor of Biological Chemistry at the University of Wollongong. He was awarded an Australian Research Council Australian Professorial Fellowship in 2008, and established UOW’s Centre for Medical and Molecular Bioscience in 2010. He is currently an academic leader of Molecular Horizons, a new facility for molecular visualization which opened in 2019.

Adelaide Almeida is a Full Professor in the Department of Biology at the University of Aveiro (Portugal), where she obtained her PhD degree in 2001 and her habilitation in 2015. She is an integrated member of the Associated Laboratory Centre for Environmental and Marine Sciences (CESAM). Recently, she has been involved in the development and application of alternative methods to the use of antibiotics, such as photodynamic therapy and phage therapy. She has published in these fields and her publications can be found at http://www.cesam.ua.pt/adelaidealmeida.

LMI CNRS UMR 5615, Université Lyon 1, Villeurbanne, France
Dr. Anthony W. Coleman studied at Huddersfield New College and qualified by competitive examination for admission to the University of Oxford. He was affiliated to Jesus College from 1972-76 obtaining an Honours degree in Chemistry. Publications 260 in Science Citation Index, 150 Invited and Plenary Talks and 22 Patents (3 licenced).

Jean-Marc SABATIER is a Director of research at the French CNRS, with PhD and HDR degrees in Biochemistry and Microbiology. He has headed several academic research teams (CNRS, INSERM and University), as well as a combined academic–industry research laboratory devoted to the engineering of therapeutic peptides (ERT62, Marseilles, France). He was also a Director of Research for several French private companies as well as a Canadian public company. He acts as a consultant for top pharmaceutical and cosmetic companies. Dr. Sabatier works in the field of animal toxins and microbes. He so far contributed to several books in toxicology and virology, and more than 180 scientific articles, 180 communications, and 55 patents in both biology and chemistry. He acts as the Editor-in-Chief of the journals Coronaviruses, Infectious Disorders – Drug Targets, and Venoms and Toxins. He is a member of 66 Editorial Boards of scientific journals, such as Peptides, Molecules, Antibiotics, Frontiers in Pharmacology, and the Journal of Biological Chemistry. He also reviewed articles submitted for publication in >100 international journals and acts as an expert for numerous national and international institutions. He won the ‘Citizen of the Year Award’ from The Nouvel Economiste (1994) for his work on antivirals. He is a member of a dozen scientific societies, such as the ‘American Peptide Society’ (charter member), ‘European Peptide Society’, ‘American Society for Microbiology’, ‘Biochemical Society’ and ‘New-York Academy of Sciences’.

Department of Clinical Microbiology, Hospital Clinic, Barcelona, Spain
Dr. Vila is currently the Head of the Department of Clinical Microbiology of the Hospital Clinic in Barcelona, Full Professor of the School of Medicine, University of Barcelona, and Research Professor in the Institute for Global Health (ISGlobal) of Barcelona, Spain. His present main field of interest is in the investigation of the molecular bases of antimicrobial resistance and the development of new drugs against bacteria and molecular tools for rapid diagnosis of infectious diseases. Dr. Vila was the Programme Director of the Congress of the European Society of Clinical Microbiology (ESCMID) from 2009 to 2014 and he has been a member of the ICAAC Scientific Program Committee (USA) for 4 years. He received an Award of the National Plan against Antibiotic Resistance (PRAN) 2018 for the Micro-combat card game, presented by the Barcelona Global Health Institute Foundation (ISGlobal) in the category of better communication and public awareness initiative on the antibiotic resistance. He is also leading the Initiative of Antimicrobial Resistance in ISGlobal. He has published 476 articles in peer-reviewed journals, 44 book chapters and directed 25 doctoral theses (number of citations, 29.536 and H-index of 84, Google Scholar). He has patented three molecules.

Department of Analytical Chemistry and Food Science, Faculty of Food Science and Technology, University of Vigo, Spain
Jesus Simal-Gandara has been a Full Professor in Food Science at the University of Vigo (Spain) since 1999 and he became the Vice-Chancellor for Internationalization in 2018. He won the 1st Spanish Award of Completion of Pharmacy and the PhD Prize from the Faculty of Pharmacy, University of Santiago de Compostela (Spain). He also was Associate Professor in 1991 at the University of Vigo. Now, he leads a research group of excellence in Northwest Spain. In addition, he was leading CIA3 (Environmental, Agricultural and Food Research Centre) formed by 10 research groups from different fields (botany, plant physiology, soil science and agricultural chemistry, biochemistry and molecular biology, nutrition and food science, biotechnology, food technology, food rheology, chemical engineering, and colloidal chemistry) from 2008 to 2018, and also was the Head of the Department of Analytical Chemistry and Food Science at the University of Vigo between 2013 and 2018. He was also Vice-Chancellor for Internationalization at the University of Vigo in 2018. His research group is specialized in chromatographic separations (GC-MS and LC-MS) and molecular biology and proteomics. They investigate the distribution of agricultural and environmental organic chemical contaminants in the food production chain, and how improving the sensory and functional quality of food, with an eye on the food chain globally, integrating environment, agriculture and food with nutrition and public health issues. His focus today is on the study of persistent organic pollutants (POPs) from the point of view of public health (epidemiology, toxicity of mixtures, metabolites, etc.), and on the study of secondary metabolites in plant foods, exploring the molecular mechanisms that explain their activity, in order to develop new nutraceuticals, functional foods and cosmetics.

School of Food Science and Engineering, South China University of Technology, Guangzhou, China
Dr. Xu obtained a B.S. and Ph.D. from South China University of Technology in 2005 and 2011, respectively, and has worked as an Assistant Professor since 2011. Dr. Xu was awarded a Top 100 National Outstanding Doctoral Dissertation in 2014. His major research interests include microbial biofilm, antimicrobial resistance, polymicrobial interaction, and rapid detection. Dr. Xu is the co-founder and co-president of Asia-Pacific Biofilms (previously known as ChinaBiofilms). Dr. Xu has published more than 100 manuscripts as first or correspondence author, with the total IF > 260, citation > 5,000, H-Index as 42, and I-10 Index as 82. Professor, South China University of Technology, Guangzhou, China. Assistant Visiting Scientist, University of Maryland, College Park, United States. Distinguished Visiting Professor, Rajamangala University of Technology Phra Nakhon, Bangkok, Thailand. Adjunct Professor, National Institute of Fundamental Science, Kandy, Sri Lanka.
Keynote Speakers

Department of Pharmacy, University “G. d'Annunzio” of Chieti-Pescara, Chieti, Italy
Prof. Simone Carradori completed his PhD in “Pharmaceutical Sciences” at the Sapienza University of Rome (Italy) and is currently an Associate Professor in medicinal chemistry at the Department of Pharmacy, “G. d’Annunzio” University of Chieti-Pescara (Italy). Prof. Carradori has collaborated and still collaborates with several departments abroad. His scientific activity is mainly focused on the synthesis and characterization of heterocyclic derivatives and the extraction/modification of natural compounds. These contributions have led to the discovery of new biologically active agents, characterized by in vitro, in vivo, and in silico studies, as well as drug repositioning in cancer and antimicrobial therapy, documented in about 212 papers in international peer-reviewed journals, one European patent, and participation in numerous conferences. Moreover, Prof. Carradori is a member of the Editorial Board of several peer-reviewed journals (Hirsch index: 42; citations: ~5852).

Department of Bioengineering, University of Pennsylvania, USA
César de la Fuente is a Presidential Assistant Professor at the University of Pennsylvania, where he leads the Machine Biology Group whose goal is to combine the power of machines and biology to help prevent, detect, and treat infectious diseases. Specifically, he pioneered the development of the first antibiotic designed by a computer with efficacy in animals, designed algorithms for antibiotic discovery, reprogrammed venoms into antimicrobials, created novel resistance-proof antimicrobial materials, and invented rapid low-cost diagnostics for COVID-19 and other infections. De la Fuente is an NIH MIRA investigator and has received recognition and research funding from numerous other groups. Prof. de la Fuente has received over 50 awards. He was recognized by MIT Technology Review as one of the world’s top innovators for “digitizing evolution to make better antibiotics”. He was selected as the inaugural recipient of the Langer Prize, an ACS Kavli Emerging Leader in Chemistry, and received the AIChE’s 35 Under 35 Award as well as the ACS Infectious Diseases Young Investigator Award. In 2021 he received the Thermo Fisher Award and the EMBS Academic Early Career Achievement Award “For the pioneering development of novel antibiotics designed using principles from computation, engineering, and biology”. Most recently, Prof. de la Fuente was awarded the prestigious Princess of Girona Prize for Scientific Research in addition to the ASM Award for Early Career Applied and Biotechnological Research. Prof. de la Fuente has given over 150 invited lectures and his scientific discoveries have yielded around 100 publications, including papers in Nature Biomedical Engineering, Nature Communications, PNAS, ACS Nano, Cell, Nature Chemical Biology, and Advanced Materials, and multiple patents.

Institute of Medical Mycology, Teikyo University, Hachioji, Tokyo, Japan
Dr. Hiroshi Hamamoto studied at the Department of Pharmaceutical Sciences, Kyushu University (1994-1998), and the Grad. Sch. Of Pharm. Sci., Kyushu University (1998-2000). He belonged to the Lab. of Microbiology and studied disinfectant-resistant strains of Staphylococcus aureus. He moved to the Grad. Sch. of Pharm. Sci. at the University of Tokyo for his doctoral studies (2000-2002), during which he established a system to evaluate the quantitative therapeutic effects of antibiotics using a silkworm-based infection system. After graduation, he worked as an assistant professor at the University of Tokyo, where he researched the analysis of pharmacokinetics in silkworm (2003-2005) and the screening of the therapeutically effective antibiotics from soil bacteria culture supernatants using a silkworm bacterial infection model. From 2005 to 2008, He successfully identified a novel antibiotic, Lysocin E, by discovering antibiotics through the use of a silkworm infection model at the Genome Pharmaceuticals Institute. He then returned to the University of Tokyo as an assistant professor. He analyzed the mechanism of lysocin E (2008-2016), finding that lysocin E has a unique mode of action that is distinct from other known antibiotics. Presently, he is an associate professor at the Teikyo University Institute of Medical Mycology. He recently determined that host–microbe interaction enhances the therapeutic efficacy of lysocin E and elucidated the detailed mechanisms involved in this finding, which have been published in Nature Communications.

Riverside University Health System-Medical Center, 26520 Cactus Avenue, Moreno Valley, CA, USA
Professor Dr. Adriana E. Rosato is a clinical microbiologist/molecular biologist with special interests in the areas of infectious diseases/antimicrobial development and resistance. Her work on infectious diseases has extended for more than 25 years, covering both the clinical and basic aspects of molecular mechanisms and the regulation of multidrug-resistant pathogens (MDR). The overarching goal of her research program is to identify novel loci involved in resistance, as well as regulatory components that will contribute to the expansion of our knowledge on the biology of MDR, which may, ultimately, provide insights into common pathways that could become new targets for chemotherapy. Moreover, her mechanistic seminal basic studies on the antimicrobial resistance of MDR have contributed to paving the way for clinical patient management, mainly for those patients who are vulnerable to recurrent MDR caused by infections for which treatment options are limited, such as cystic fibrosis, cancer, and recalcitrant bacteremia. Her research has been continuously funded by NIH-NIAID, pharmaceutical agencies, and numerous foundations. She has invested a great amount of effort into contributing to and advising the field of antimicrobial resistance by participating in the revision of scientific advances and discoveries at the NIH and European Agencies; through this, she has had the opportunity to contribute to important decisions regarding funding and studies in the area of antimicrobial resistance. She is an advisor consultant of pharmaceutical companies and the WHO division of antimicrobial resistance. Moreover, over the years, she has shown a strong commitment to mentoring young investigators, and has served and is currently serving as a primary mentor for several individuals. Aside from her activities, in the last two years, she has led the efforts of the COVID-19 pandemic at the Riverside University Health System, Medical Center, Riverside, CA, from diagnostics, genomics, the evolution of COVID-19 variants to research.

UCL School of Pharmacy, University College London, London WC1N 1AX, UK
Mire Zloh was a Visiting Professor at the UCL School of Pharmacy until January 2021, when he took position of a Teaching Fellow at the same institution. He is an Emeritus Professor of Medicinal Chemistry with University of Hertfordshire, where he was the Head of Pharmaceutical Chemistry and Research Professor from January 2013 until September 2017. He also acts as Innovation Advisor to the Dean of the Faculty of Pharmacy, University Business Academy in Novi Sad Serbia. His research interests are in the areas of computer-aided molecules, antimicrobial peptides, and formulation design and structural chemistry. Most notably, Prof. Zloh proposed that modulators of multidrug resistance might form complexes with substrates of efflux pumps to act as “escort” molecules and deliver drugs to the site of action, which may provide an additional route in the design of adjuvant therapies to treat infections by resistant bacteria. In efforts to develop polymer-based drug delivery systems, a method was develop to translate monomeric linear sequences into a full atomistic model of a hyperbranched molecule. His research resulted in over 125 peer-reviewed articles (h- index 31).

Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties, University of Palermo, Palermo, Italy
Dr Fasciana (PhD, and microbiologist) is an under-40 researcher with extensive experience in the culture of bacteria and yeasts. She collaborated with a scientific project at the University of Palermo from 2010 on the quality of research. Since November 2019, Dr Fasciana has been appointed as an Assistant Professor at the Department of Health Promotion, Mother and Child Care, Internal Medicine, and Medical Specialties University of Palermo. Her current research interests include antimicrobial resistance, medical biofilms, and in vitro studies on the antibacterial activity of new drugs.

Department of Molecular Biology, University of Gdańsk, Gdańsk, Poland
Grzegorz Wegrzyn graduated from the University of Gdansk, Poland. In 1987 he obtained an MSc degree in biology, and in 1991 a PhD degree in molecular genetics. His PhD thesis was focused on the regulation of DNA replication in starved cells. Then, in 1991, he was a research fellow at the Department of Biochemistry, University of Nottingham Medical School (UK), where he worked on the mechanisms of gene expression regulation in bacteria. In 1992 he was a post-doctoral researcher at the Center for Molecular Genetics, University of California at San Diego (USA), where he investigated the regulation of viral DNA replication. Since 1996 he has been a Professor and the Head of the Department of Molecular Biology at the University of Gdansk (Poland). In his laboratory, several projects are being conducted, focused mainly on the regulation of gene expression and DNA replication, the control of bacteriophage development, the use of bacteriophages in biotechnological applications, and the mechanisms and new treatment methods of human genetic and neurodegenerative diseases. Grzegorz Wegrzyn has been a co-author of over 400 scientific articles in peer-reviewed journals and over 600 communications for scientific conferences. He has supervised 54 PhD theses, and led over 30 research projects with both national and international grants. He is a member of the American Society for Biochemistry and Molecular Biology, American Society for Microbiology, Society for Experimental Biology and Medicine, and International Society for Plasmid Biology. He is an editor of several scientific journals, including FEMS Microbiology Reviews, Microbial Cell Factories (Editor-in-Chief), Metabolic Brain Disease (Deputy-Chief-Editor), Plasmid, Oxidative Medicine and Cellular Longevity, Scientific Reports (Senior Editor), and Acta Biochimica Polonica (Editor-in-Chief).

School of Chemistry and Molecular Bioscience, University of Wollongong, Wollongong, Australia
Aaron Oakley graduated with a PhD from the University of Melbourne in 1997. He helped to establish a new structural biology laboratory at the University of Western Australia from 1998 to 2003, solving a wide range of structures from mosquito-detoxifying enzymes responsible for pesticide resistance to bacterial dehalogenases for bioremediation applications. He was appointed Fellow at the Research School of Chemistry at the Australian National University in 2004. After a two-year interlude at the Division of Molecular and Health Technologies in the Commonwealth Scientific and Industrial Research Organisation (2008–2009), he was in 2010 awarded an ARC Future Fellowship to establish structural biology at the University of Wollongong. The laboratory moved to the new Molecular Horizons Institute in that university in 2019.

Helen Zgurskaya, Ph.D., M.S., is a George Lynn Cross professor of chemistry and biochemistry at the University of Oklahoma, where she leads a research program on antibiotic discovery and resistance, multidrug efflux mechanisms, and efflux pump inhibitors. She is a founder of the university’s Center for Antibiotic Resistance and Discovery, an international team composed of 13 research groups spread across five academic campuses and the Oak Ridge and Los Alamos national laboratories. Zgurskaya has written more than 80 papers, several book chapters and is a co-editor of a book. She is also an associate editor of the ACS Infectious Diseases journal and a member of the editorial board of the Antimicrobial Agents and Chemotherapy and Frontiers in Microbiology journals. She received a master’s degree in microbiology from Dnepropetrovsk State University, Ukraine, a doctorate in microbiology from the Russian Academy of Sciences, Russian Federation, and was a postdoctoral researcher at the Stanford University School of Medicine and the University of California, Berkeley.

Department of Biological Engineering Massachusetts Institute of Technology; Wyss Institute, Harvard University; Broad Institute of MIT and Harvard, Cambridge MA, USA
Jim Collins is the Termeer Professor of Medical Engineering and Science and Professor of Biological Engineering at MIT, as well as a Member of the Harvard-MIT Health Sciences and Technology Faculty. He is also a Core Founding Faculty member of the Wyss Institute for Biologically Inspired Engineering at Harvard University and an Institute Member of the Broad Institute of MIT and Harvard. He is one of the founders of the field of synthetic biology, and his research group is currently focused on using synthetic biology to create next-generation diagnostics and therapeutics. Professor Collins’ patented technologies have been licensed by over 25 biotech, pharma, and medical devices companies, and he has co-founded a number of companies, including Synlogic, Senti Biosciences, Sherlock Biosciences, and Cellarity, as well as Phare Bio, a non-profit focused on AI-driven antibiotic discovery. He has received numerous awards and honors, including a Rhodes Scholarship and a MacArthur “Genius” Award, and he is an elected member of all three national academies—the National Academy of Sciences, the National Academy of Engineering, and the National Academy of Medicine.

Faculty of Medicine and Dentistry and University Hospital Olomouc, Palacky University, Olomouc, Czech Republic
Milan Kolář, MD, PhD is currently the head of the Department of Microbiology, University Hospital Olomouc, Czech Republic, and a full professor at the Faculty of Medicine and Dentistry, Palacký University Olomouc. He obtained his PhD degree in 1999 and habilitation in 2001 at Palacký University Olomouc. In 2007, he was appointed Professor of Medical Microbiology at Masaryk University, Czech Republic. His scientific activity is mainly focused on bacterial infections, antimicrobial resistance, antibiotic treatment, and development of new antibacterial drugs. He is the first author or co-author of 160 scientific publications indexed in the Web of Science, with a citation rate of 5419 and an h-index of 29. Prof Kolář is a member of editorial boards of several peer-reviewed journals (including Antibiotics and Life) and the editor-in-chief of the Czech journal Clinical Microbiology and Infectious Diseases. Further, he is the chair of the Czech Medical Association’s Society of Physicians in Olomouc and the scientific secretary of the Czech Medical Association’s Society for Medical Microbiology. More information is available at: https://mikrobiologie.fnol.cz/.

Microbiology Unit, Reina Sofia University Hospital, IMIBIC, Department of Agricultural Chemistry, Edafology and Microbiology, University of Cordoba, Cordoba, Spain
Prof. Luis Martínez Martínez is Head of the Unit of Clinical Microbiology at Reina Sofia University Hospital (RSHU) and Titular Professor of Microbiology at the School of Medicine of the University of Cordoba. He leads the IMIBIC research group “Clinical and Molecular Microbiology”. He is the President of the Andalusian Society of Microbiology and Parasitology (SAMPAC) and has been the Secretary of the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). He acted, from 2011 to 2020, as President of the Spanish Committee of the Antibiogram (COESANT), from 2012 to 2014 as member of the Executive Committee of EUCAST, and from 2013 to 2016 as a member of the Scientific Committee on Emerging and Newly Identified Heath Risks (SCENIHR), European Commission, DG Health and Consumers. Prof. Martínez Martínez has supervised 13 PhD theses. He is a member of numerous national and international evaluation panels. He has served as Editor of Enfermedades Infecciosas y MiIcrobiologia Clínica and is a reviewer for multiple peer-review journals. Prof. Martínez Martínez has considerable experience in the fields of antimicrobial resistance and new diagnostic methods in clinical microbiology, with 398 scientific publications, over 12,600 citations and an h-index of 62. He has been awarded 25 projects in competitive national and international funding. He has given over 160 talks as an invited speaker and received over 50 invitations to chair scientific sessions and has been a member of organizing committees for international and national meetings.

School of Cancer and Pharmaceutical Science, King's College London, London, UK
Miraz Rahman is a Professor of Medicinal Chemistry in the School of Cancer and Pharmaceutical Sciences, King’s College London. After working three years in the industry, he went back to academia and obtained his PhD from the School of Pharmacy (currently UCL School of Pharmacy) in synthetic medicinal chemistry. His research activities are focused on the application of advanced computational chemistry, synthetic medicinal chemistry and chemical biology techniques to the design, synthesis and evaluation of novel drug-like chemical scaffolds as anticancer and anti-infective agents. He joined King’s College London in 2012 and currently holds the position of Chair in Medicinal Chemistry and Antimicrobial Research Theme Lead of Institute of Pharmaceutical Science. He is working with the UK Health Security Agency to develop new-generation, efflux-resistant antimicrobials and new chemical tools to study antimicrobial resistance (AMR). His research group has invented new classes of efflux-resistant antibiotics and antifungal agents that are currently being commercialised. Professor Rahman has experience in both early- and late-stage drug discovery and translational research, co-founding Transcriptogen Ltd (2013), Femtogenix Ltd (2015) and the antibiotic company Necobiotix Ltd (2020).

Department of Civil and Environmental, University of Maryland, College Park, MD, USA
Dr. Junyan Liu is a research associate and postdoctoral fellow at the University of Maryland, College Park. She obtained her Ph.D. degree from South China University of Technology in 2019 and studied at the University of Tennessee Health Science Center from 2016 to 2017. In 2019, she was appointed a Distinguished Visiting Scientist at Rajamangala University of Technology Phra Nakhon in Thailand. Her major research fields include microbial biofilms, antimicrobial resistance, the VBNC state, and polymicrobial interaction. She has published 42 papers as the first or corresponding author, with a total impact factor of 160. She was awarded the Bronze Prize at the first National Postdoctoral Innovation and Entrepreneurship Competition in 2021; was heralded as the “Outstanding Young Investigator Award in Biofilms” by Antibiotics (IF 4.639); and was the 2020 Postdoc Research Showcase 2nd Place Winner in Tennessee, USA. She is also an Editorial Board Member of the journals Annotations and Bioengineered and a Guest Editor for 6 journals.

Department of Biomedical Engineering and Chemical Engineering, The University of Texas at San Antonio, San Antonio, TX, USA
Nehal I. Abu-Lail received her B.S. and M.S. degrees in Chemical Engineering from Jordan University of Science and Technology. She earned her Ph.D. in Chemical Engineering from Worcester Polytechnic Institute in 2004. She has been an Associate Professor at the University of Texas San Antonio in the Department of Biomedical Engineering and Chemical Engineering since August 2018. Prior to that, she was an associate professor at the Gene and Linda Voiland School of Chemical Engineering and Bioengineering at Washington State University. Her research is focused on the fundamental understanding of physiochemical cellular properties and interactions in environmental and biomedical systems. She has published over 60 technical articles and presented her research in over 200 national meetings. She is currently teaching the courses “Transport Phenomena I and II” in Chemical Engineering.
List of Keynotes & Videos
Keynotes
Speaker: Nehal I. Abu-Lail
Presentation Title: Atomic force microscopy investigations of the outer membranes of persister multidrug resistant (MDR) Escherichia coli
Live Sessions - Registration and Program
Welcome to the ECA2022 Live Sessions! You are invited to register online.
There will be four live sessions, all of which are completely free to attend. There is a different registration link for each live session. You only need to register for the ones that you wish to join. If you would like to attend more than one live session, please register for each session separately.
Authors who are providing submissions for ECA2022 will have priority when registering (at no extra cost) for the live online sessions with our keynote speakers. If the session is not completely full, the registration will be opened for unregistered participants. Registrations with academic institutional email addresses will be prioritized.
After registering, you will receive a confirmation email about joining the webinar. The number of participants who can be admitted to a live session is limited, but the recording will be made available on Sciforum shortly afterwards.
-
Live session 1 is on 23 June 2022 at 1:00pm CEST.
First Day: 23 June 2022 (Europe and UK)
Webinar Chair: Jordi Vila
Schedule: 2h 30min (Total)
|
Speaker |
Schedule (CEST) |
Schedule (UK) |
Time Slot |
Title |
|
Chair |
13:00 |
12:00 |
10 min |
Opening Ceremony |
|
Luis Martínez-Martínez |
13:10 |
12:10 |
30 min |
Mechanisms of resistance in Gram-negative bacteria to new beta-lactam/beta-lactamase inhibitor combinations |
|
|
13:40 |
12:40 |
5 min |
Q&A |
|
Mire Zloh |
13:45 |
12:45 |
30 min |
Small cationic peptides as potentiating agents against Susceptible and multi-drug resistant Escherichia coli |
|
|
14:15 |
13:15 |
5 min |
Q&A |
|
Simone Carradori |
14:20 |
13:20 |
30 min |
Exploring new derivatives of secondary metabolites as inhibitors of Helicobacter pylori and anti-biofilm agents endowed with low cytotoxicity on human cells |
|
|
14:50 |
13:50 |
5 min |
Q&A |
|
Miraz Rahman |
14:55 |
13:55 |
30 min |
Innovations in developing antimicrobials in the age of antimicrobial resistance |
|
|
15:25 |
14:25 |
5 min |
Q&A |
-
Live session 2 is on 24 June 2022 at 5:00pm CEST.
Second Day: 24 June 2022 (USA)
Webinar Chair: Marc Maresca
Schedule: 2h 20min (Total)
|
Speaker |
Schedule |
Schedule |
Schedule |
Time Slot |
Title |
|
James Collins |
11:00 |
10:00 |
8:00 |
30 min |
The Antibiotics-AI Project |
|
|
11:30 |
10:30 |
8:30 |
5 min |
Q&A |
|
Cesar de la Fuente-Nunez |
11:35 |
10:35 |
8:35 |
30 min |
Machine Biology for Infectious Diseases |
|
|
12:05 |
11:05 |
9:05 |
5 min |
Q&A |
|
Helen Zgurskaya |
12:10 |
11:10 |
9:10 |
30 min |
Making sense of drug efflux transporters in the physiological environment |
|
|
12:40 |
11:40 |
9:40 |
5 min |
Q&A |
|
Adriana E. Rosato |
12:45 |
11:45 |
9:45 |
30 min |
Unveiling Mechanism and Regulation of Ceftaroline Resistance in Cystic Fibrosis MRSA infections |
|
|
13:15 |
12:15 |
10:15 |
5 min |
Q&A |
-
Live session 3 is on 27 June 2002 at 3:00am CEST.
Third Day: 27 June 2022 (Asia, USA, and Australia)
Webinar Chair: Zhenbo Xu
Schedule: 1h 45min (Total)
|
Speaker |
Schedule (CN) |
Schedule (JP) |
Schedule (USA MD) |
Schedule (AU) |
Time Slot |
Title |
|
Junyan Liu |
9:00 |
10:00 |
21:00 |
12:00 |
30 min |
Epidemiology of ESKAPE in Southern China for 15 years |
|
|
9:30 |
10:30 |
21:30 |
12:30 |
5 min |
Q&A |
|
Aaron J. Oakley |
9:35 |
10:35 |
21:35 |
12:35 |
30 min |
Bacterial protein structure prediction |
|
|
10:05 |
11:05 |
22:05 |
13:05 |
5 min |
Q&A |
|
Hiroshi Hamamoto |
10:10 |
11:10 |
22:10 |
13:10 |
30 min |
The usefulness of silkworm in the screening of antimicrobial agents |
|
|
10:40 |
11:40 |
22:40 |
13:40 |
5 min |
Q&A |
-
Live session 4 is on 28 June 2022 at 10:00am CEST.
Fourth Day: 28 June 2022 (Europe and UK)
Webinar Chair: Anthony William Coleman
Schedule: 2h 20min (Total)
|
Speaker |
Schedule (UK) |
Schedule (CEST) |
Time Slot |
Title |
|
Milan Kolar |
9:00 |
10:00 |
30 min |
AMR and antibiotic therapy in COVID-19-positive patients in ICU |
|
|
9:30 |
10:30 |
5 min |
Q&A |
|
Grzegorz Wegrzyn |
9:35 |
10:35 |
30 min |
Antimicrobial Activities of Compounds Produced by Newly Isolated Streptomyces Strains from Mountain Caves |
|
|
10:05 |
11:05 |
5 min |
Q&A |
|
Teresa Fasciana |
10:10 |
11:10 |
30 min |
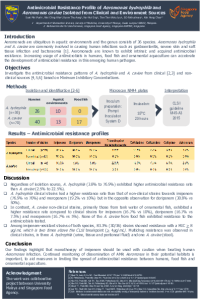
Antibiotic resistance profiles in Helicobacter pylori strains isolated in Sicily (Italy) |
|
|
10:40 |
11:40 |
5 min |
Q&A |
Live Session Recordings

List of accepted submissions (66)
| Id | Title | Authors | Presentation Video | Poster PDF | |||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sciforum-057844 | In vitro antifungal activity of Boesenbergia rotundo Linn. and Syzygium aromaticum L. Merr. & Perry. extracts against Aspergillus flavus | , | N/A |

|
Show Abstract |
||||||||||||||||||||||||||||||||||||
|
Aspergillus flavus is a common human pathogen that releases mycotoxin into the host and is frequently treated with synthetic fungicides, but the fungicides have serious human health consequences. Natural products derived from higher plant species have long been investigated as a potential means of controlling pathogenic microorganisms. The indigenous vegetables Boesenbergia rotunda and Syzygium aromaticum are widely distributed in the tropical area. These plants have also been reported in traditional uses for the antimicrobial activity. The purpose of the study was to explore the antifungal susceptibility of dichloromethane and ethanol extracts of B. rotunda rhizomes and S. aromaticum flower buds by Soxhlet’s apparatus against A. flavus using the poison food technique. The effective extract was also subjected to preliminary phytochemical screening tests. The experiment used a completely randomized design with triplications. B. rotunda ethanol extract demonstrated significantly higher potential antifungal activity. The values of minimum inhibitory concentration (MIC) and minimum fungicidal concentration (MFC) of B. rotunda ethanol extract were 6.25 and 50 mg/ml, respectively, when tested using the macro-dilution method. According to phytochemical tests, the ethanol extract also contained alkaloids, flavonoids, cardiac glycosides, and saponins. The study suggests that a basic guideline for using this as an effective antifungal compound should be separated from the B. rotunda ethanol extract in the future for topical anti-pathogenic fungus. |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-059337 | In silico screening and in vitro validation of natural-based LuxS inhibitors |
Susana Fernandes ,
,
,
,
|
N/A | N/A |
Show Abstract |
||||||||||||||||||||||||||||||||||||
|
Quorum sensing (QS) system is related to cell to cell communication as a function of population density, which regulates many physiological functions including biofilm formation and virulence gene expression. The interspecies communication is mediated by autoinducer-2 (AI-2) that is catalysed by LuxS from S-ribosylhomocysteine (RHC). QS inhibitors have emerged as a promising strategy for biofilm and virulence attenuation, which improves the potential of antimicrobial treatments by increasing microbial susceptibility. Among a wide variety of phytochemicals, many of them have been described as QS inhibitors. Driven by the promising phytochemicals clues, this study aimed to identify new active plant natural compounds against LuxS through in silico analysis, followed by in vitro validation. A representative strain for the study of LuxS inhibition, Bacillus subtilis, and a reporter strain sensitive to AI-2, Vibrio harveyi BB170, were used. Firstly, the optimization of a virtual screening protocol was conducted by applying a combination of protein-ligand docking, receptor-based virtual screening, and free energy calculations. Then, optimized virtual screening protocols were applied to screen a phytochemical database containing 3479 drug-like compounds. Based on binding energy scores and compounds cost, the most promising and selected phytochemicals were curcumin, pioglitazone hydrochloride, and 10-undecenoic acid. According to the obtained scores, 10-undecenoic acid could strongly interact with the active binding site of LuxS, while curcumin and pioglitazone hydrochloride had a slight probability compared to natural ligand (RHC). In vitro analysis corroborated the QS inhibitory activity of curcumin and 10-undecenoic acid, however, pioglitazone hydrochloride had no relevant effect. Curcumin (1.25-5 µg/mL) triggered 33-77% reduction of AI-2 accumulation and 10-undecenoic acid (12.5-50 µg/mL) reduced 36-64%. In conclusion, in silico analysis allowed the identification of LuxS antagonistic phytochemicals, revealing curcumin and 10-undecenoic acid as active QS inhibitors. Additionally, this study highlighted that although computational screening is a powerful and quick tool to identify lead compounds, in vitro validation is needed to guarantee high levels of accuracy. |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-059383 | Methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus pseudintermedius (MRSP) in skin infections from company animals in Portugal (2013-2021) | , , , , , | N/A |

|
Show Abstract |
||||||||||||||||||||||||||||||||||||
|
The increasing of multiresistant bacteria worldwide is one of the great concerns in both human and veterinary medicine. Bacterial dermatitis is a frequent problem in small animals, with the primary skin pathogens being Staphylococcus species. Treatment has been made more difficult by the emergence of antibiotic resistance in staphylococcal bacteria in the form of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus pseudintermedius (MRSP). The study aimed to investigate MRSA and MRSP in pyodermas admitted to the INNO Veterinary Laboratory (Braga, Portugal), in the period 2013-2021. All microbiological cultures from skin infections from dogs and cats submitted to the INNO Veterinary Laboratory between January 2013 to June 2021 were considered. For the study, only samples with S. aureus and S. pseudintermedius growth were selected. In those two agents’ methicillin resistance was phenotypically detected in the laboratory by MIC determination and by disc diffusion to oxacillin. From a total of 730 samples that tested positive for bacterial growth, 101 (13,8%) were S. pseudointermedius and 27 (3,7%) were S. aureus. The isolates tested for oxacillin 9% (n=6) were MRSP and 6% (n=4) MRSA. The results obtained in this study helps to understand the situation at a national level, where studies in this area are almost non-existent. The presence of MRSA or MRSP in small animals indicates that they are part of the animal-human-environment transmission 'triangle', which should lead us to think of this issue as a public health problem. |
|||||||||||||||||||||||||||||||||||||||||
| sciforum-059843 | Antibiotic Prescriptions in Pediatric Patients Hospitalized with Pneumonia at a University Hospital | , , , , , , | N/A | N/A |
Show Abstract |
||||||||||||||||||||||||||||||||||||
|
Community-acquired pneumonia (CAP) is the leading cause of hospitalization in the Brazilian Unified Health System, with a mortality rate of 18% in children under 5 years of age. Therefore, there is a need for an effective treatment, including antibiotic therapy, based on the main causative agents of the infection. However, there is a risk of the development of bacterial resistance, making it necessary to monitor this use in order to reduce the speed of emergence of multidrug-resistant strains. Thus, this study aims to verify the profile of antibiotic use in children and adolescents treated at a brazilian university hospital. The research consists of a cross-sectional retrospective and descriptive study based on data obtained from medical records provided by the institution, after approval by the ethics committee, and organized in Excel spreadsheets, covering the period from September 2017 to December 2020. It was observed that the profile of this group of patients consists of: a female prevalence in 2017 and 2020 (59% and 57% respectively); while in the years 2018 and 2019, males were higher, 52% and 59%. Regarding age, the age group from 3 months to 4 years was predominant (59.64%). Regarding the use of antibiotics by age group, the following data were found: up to 3 months, the most used were ampicillin (44%) and azithromycin (24.25%); from 4 months to 4 years, ampicillin (32.9%), ceftriaxone (31.7%) and azithromycin (25.9%); and over 5 years, ceftriaxone (33.8%), ampicillin (29.95%) and azithromycin (22.22%). Thus, when comparing the profile found with that recommended by the protocol adopted by the hospital, we can conclude, with the data analyzed, that there is a negligence in the prescription of antimicrobials in the treatment of pediatric CAP, which may corroborate the growth of bacterial resistance, longer hospital stay and, as a result, greater expenditure on care and reduced favorable clinical outcome. |
|||||||||||||||||||||||||||||||||||||||||
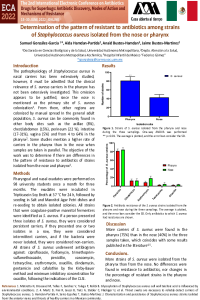
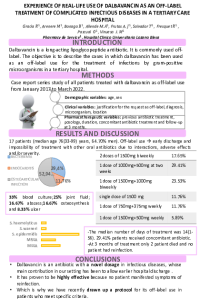
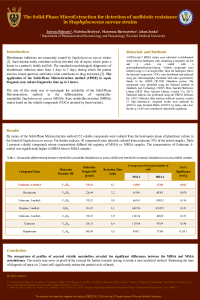
| sciforum-060033 | In vitro activity of ceftazidime-avibactam against ESBL producing and carbapenem-resistant Gram – negative bacteria recovered from blood and fecal samples of patients after hematopoietic stem-cell transplantation | , , , | N/A |
|
Show Abstract |
||||||||||||||||||||||||||||||||||||
|
Patients, receiving hematopoietic stem-cell transplantation (HSCT) are prone to develop invasive infections due to disease and transplantation-related immunosuppression. The main causative agents often originate from the digestive tract and are multidrug resistant. Our aim was to investigate the in vitro activity of ceftazidime-avibactam (CZA) against extended spectrum beta-lactamase (ESBL) - producing and carbapenem – resistant (CR) Gram – negative bacteria recovered from blood and fecal samples of patients following HSCT, hospitalized in University Hospital “Saint Marina” - Varna during 2019-2021. A total of 48 isolates (E. coli, n=20; Enterobacter cloacae, n=9; Klebsiella pneumoniae, n=6; Serratia marcescens, n=1; Acinetobacter baumannii, n=2; Pseudomonas putida, n=4; Pseudomonas aeruginosa, n=4; Pseudomonas mendocina, n=1; Pseudomonas composti, n=1) were studied. MALDI Biotyper Sirius (Bruker, Germany) and the automated Phoenix system (BD, USA) were used for species identification and susceptibility testing. Twenty four isolates, included in this study, were resistant to third and fourth generation cephalosporins and therefore identified as ESBL producers (E. coli, n=12; E. cloacae, n=7; K. pneumoniae, n=4; S. marcescens, n=1). A multiplex PCR was used for genes detection, associated with carbapenem resistance. In the studied group, eleven isolates (23%) were CR (E. cloacae, n=1; Pseudomonas spp., n=8; A. baumannii, n=2). All 24 ESBL producing isolates were CZA susceptible. In the group of CR isolates, only 1 P. aeruginosa was susceptible to CZA, while 10 CR isolates were resistant. Genes associated with class B and class D carbapenemases were detected by PCR (blaVIM and blaOXA-like). In conclusion, in our study all ESBL producers were susceptible to CZA, while 91% of the CR isolates (all class B and class D carbapenemase producers) were resistant. CZA is a drug combination that is highly active against ESBL producers but its spectrum of activity is limited against carbapenemase producers. Therefore other novel antimicrobial agents are urgently needed. |
|||||||||||||||||||||||||||||||||||||||||
Call for Submissions
The 2nd International Electronic Conference on Antibiotics
The 2nd International Electronic Conference on Antibiotics—Drugs for superbugs: Antibiotic discovery, modes of action and mechanisms of resistance (ECA2022), chaired by Prof. Dr. Manuel Simões and Prof. Dr. Jesus Simal-Gandara, will be held on eca2022.sciforum.net from 15th–30th June 2022.
This conference aims to provide an opportunity to representatives of academia, industry and health services to discuss and advance our current knowledge on how to control microorganisms. We hope to encourage discovery across the discipline as we cover the following seven broad themes in Sessions S1–S7, as listed below:
-
Antimicrobial Discovery, Development & Optimization;
-
Mechanisms of Action & Resistance;
-
Epidemiology & Multidrug Resistance;
-
Repurposing & Antimicrobial Adjuvants;
-
Antimicrobials & Interfaces;
-
Clinical Studies;
-
Initiatives and Policies on Antimicrobial Development and Resistance.
We are pleased to invite the global community of scholars to join ECA2022 to present their latest antibiotics research and development and share novel ideas on the multidisciplinary aspects of the research and development of antibiotics and antimicrobial resistance-related topics. Thanks to the flexibility of our innovative electronic platform, you are welcome to both upload and present your work and to attend the conference completely free of charge. We are also considering creating a Special Issue for selected conference papers in our journal Antibiotics (ISSN 2079-6382, IF 4.639). Papers published in this Special Issue, should one be launched, would receive a 20% discount on the article processing charge.
ECA2022 offers you the opportunity to participate in an international scholarly conference without the concerns and expense of traveling—all you need is access to the Internet. During the conference period, you will be able to upload papers, posters, presentations (including videos) and comment on other presentations, as well as engage with fellow scholars in real-time. In this way, the conference offers a novel opportunity to exchange opinions and views within the scholarly community and to discuss the papers and latest research in a discussion forum.
Abstracts (in English) should be submitted online by clicking the "Submit Abstract" at the top of the web page, or submitted HERE before 15th April 2022. After abstracts are accepted, please submit the full Proceeding Paper (Or Poster/Video) by 10th May 2022. The conference itself will be held from 15th–30th June 2022.
We hope that you will be able to join this exciting event which is organized and sponsored by MDPI, a scholarly open access publisher (https://www.mdpi.com/).
Paper Submission Guidelines:
For information about the procedure for the submission, peer-review, revision and acceptance of conference proceedings papers, please refer to the section "Instructions for Authors" at eca2022.sciforum.net.
Timeline:
Abstract Submission: 15th April 2022
Notification of Acceptance: 30th April 2022
Proceedings Paper Submission Deadline: 10th May 2022
Conference Date: 15th–30th June 2022
We look forward to receiving your research papers and to welcoming you to the electronic conference.
Instructions for Authors
Submissions should be be done by the authors online at eca2022.sciforum.net by registering and logging in to this website.
-
Scholars interested in participating in this conference can submit an abstract (about 200–300 words covering the areas of manuscripts for the proceeding issue) on this website until 15th April 2022.
-
The Conference Committee will evaluate the submitted abstract. All authors will be notified by 30th April 2022 about the acceptance of their abstract.
-
If the abstract is accepted, the author is asked to submit a manuscript (short proceeding paper, 3–6 pages), and/or a poster before the submission deadline of 10th May 2022. Optionally, authors of accepted abstracts will be able to submit a slides presentation (in PDF) and/or a short video presentation (3–5 minutes) as supporting material.
-
The manuscripts and presentations will be available on eca2022.sciforum.net for discussion during the time of the conference 15th–30th June 2022. And the accepted proceeding papers will probably be published in Journal Medical Sciences Forum (You will be informed before your abstract is published, you can decide whether to publish it on Medical Sciences Forum). Publication of proceeding paper is free of charge.
-
All participants of ECA2022 are welcome to submit an extended full paper to our Special Issue in the journal Antibiotics. The submission to the journal is independent from the conference proceedings and will follow the usual process of the journal, including peer-review, Article Processing Charges (APC), etc. Papers presented at the conference will be granted a 20% discount on the APC in the Special Issue.
Manuscripts (proceeding papers) for the proceeding issue must have the following organization:
First page:
- Title
- Full author names
- Affiliations (including full postal address) and authors' e-mail addresses
- Abstract (200–250 words)
- Keywords
- Introduction
- Methods
- Results and Discussion
- Conclusions
- (Acknowledgements)
- References
Manuscripts should be prepared in MS Word or any other word processor and should be converted to the PDF format before submission. The publication format will be PDF. The manuscript should count at least 3 pages (incl. figures, tables and references) and should not exceed 6 pages.
-
Formatting / Style: The paper style of the Journal Medical Sciences Forum should be followed. You may download the template file to prepare your paper. Accepted file formats are:
MS Word: Manuscript prepared in MS Word must be converted into a single file before submission. When preparing manuscripts in MS Word, the ECA2022 Microsoft Word template file (see download below) must be used. Please do not insert any graphics (schemes, figures, etc.) into a movable frame which can superimpose the text and make the layout very difficult.
LaTeX: Manuscripts prepared in LaTeX must be collated into one ZIP folder (include all source files and images, so that the Conference Secretariat can recompile the submitted PDF). When preparing manuscripts in LaTeX, please use the ECA2022 LaTeX template files.
-
Authors List and Affiliation Format: Authors' full first and last names must be given. Abbreviated middle name can be added. For papers written by various contributors a corresponding author must be designated. The PubMed/MEDLINE format is used for affiliations: complete street address information including city, zip code, state/province, country, and email address should be added. All authors who contributed significantly to the manuscript (including writing a section) should be listed on the first page of the manuscript, below the title of the article. Other parties, who provided only minor contributions, should be listed under Acknowledgments only. A minor contribution might be a discussion with the author, reading through the draft of the manuscript, or performing English corrections.
-
Figures, Schemes and Tables: Authors are encouraged to prepare figures and schemes in color. Full color graphics will be published free of charge. Figure and schemes must be numbered (Figure 1, Scheme I, Figure 2, Scheme II, etc.) and a explanatory title must be added. Tables should be inserted into the main text, and numbers and titles for all tables supplied. All table columns should have an explanatory heading. Please supply legends for all figures, schemes and tables. The legends should be prepared as a separate paragraph of the main text and placed in the main text before a table, a figure or a scheme.
-
References: The full titles and the cited papers must be given. Reference numbers should be placed in square brackets [ ], and placed before the punctuation; for example [4] or [1–3], and all the references should be listed separately and as the last section at the end of the manuscript.
-
Submission: Manuscripts should be submitted online at eca2022.sciforum.net by registering and logging in to this website.
Slides---Authors are encouraged to prepare a presentation in PowerPoint or similar software, to be displayed online along with the Manuscript. Slides can be prepared in exactly the same way as for any traditional conference where research results can be presented. Slides should be converted to the PDF format before submission so that our process can easily and automatically convert them for online displaying.
Video---Authors are also encouraged to submit video presentations. This is a unique way of presenting your paper and discussing it with peers from all over the world.
• The video should be no more than 250Mb and be prepared with the following formats: mp4 / webm / ogg.
• The video should be submitted before 10th May 2022.
Posters will be available on this conference website during and after the event. Like papers presented on the conference, participants will be able to ask questions and make comments about the posters. Posters can be presented without an accompanying proceedings paper.
After acceptance, please upload a copy of the proceedings/abstract as a PDF and word, in the corresponding fields, and upload the Poster PDF in the field "Presentation PDF (optional)".
1)The poster should be in PDF format
2)The minimum size for images is 148 mm × 210 mm (horizontal × vertical) at 300 dpi.
3)The content of the poster should be a comprehensive presentation of your accepted submission.
4) No copyright issues with any elements in the poster.
For detailed instructions on how to submit a poster, please contact us at eca2022@mdpi.com.
It is the authors' responsibility to identify and declare any personal circumstances or interests that may be perceived as inappropriately influencing the representation or interpretation of clinical research. If there is no conflict, please state here "The authors declare no conflict of interest." This should be conveyed in a separate "Conflict of Interest" statement preceding the "Acknowledgments" and "References" sections at the end of the manuscript. Financial support for the study must be fully disclosed under "Acknowledgments" section.
MDPI, the publisher of the Sciforum.net platform, is an open access publisher. We believe that authors should retain the copyright to their scholarly works. Hence, by submitting a Communication paper to this conference, you retain the copyright of your paper, but you grant MDPI the non-exclusive right to publish this paper online on the Sciforum.net platform. This means you can easily submit your paper to any scientific journal at a later stage and transfer the copyright to its publisher (if required by that publisher).
Event Awards
To acknowledge the support of the conference esteemed authors and recognize their outstanding scientific accomplishments, we are pleased to launch the following awards:

The Best Paper Award has been awarded to
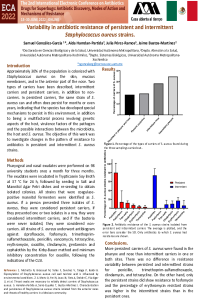
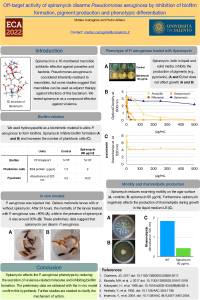
- sciforum-060875 "Off-Target Activity of Spiramycin Disarms Pseudomonas aeruginosa by Inhibition of Biofilm Formation, Pigment Production and Phenotypic Differentiation"
By Matteo Calcagnile and Pietro Alifano

The Best Poster Award has been awarded to
- sciforum-060843 "Tackling Multi-Drug Resistance in Pseudomonas aeruginosa Thanks to a New Promising Anti-virulence Strategy"
By Elodie Lohou, Marie Hanot, Marine Duplantier, Céline Dalle, Nicolas Taudon and Pascal Sonnet
The Awards
Number of Awards Available: 1
Prize: 500 CHF and a chance to publish a waived paper in Antibiotics in 2022.Number of Awards Available: 1
Prize: 300 CHF.Terms and Conditions:
1. Full paper/poster must be submitted to ECA2022.
2. The quality of the paper/poster.
3. The scientific content of the paper/poster.
Evaluation
1. Each Evaluation Committee member will give an assessment for each paper/poster in terms of the criteria outlined above.
2. The score for each paper/poster will be ranked, from highest to lowest.
3. If two or more papers/posters get the same score, further evaluation will be carried out.
4. All decisions made by the Evaluation Committee are final.
Conference Secretary
Ms. Lois Liu
Ms. Kristine Wang
MDPI Branch Office, Beijing
E-mail: eca2022@mdpi.com
Sponsors and Partners
For information regarding sponsorship and exhibition opportunities, please click here.
Organizers
Media Partners
S1. Antimicrobial Discovery, Development & Optimization
Session Chair
Dr. Marc Maresca, CNRS, Aix-Marseille University, Centrale Marseille, iSm2, Marseille, France
Show all published submissions (24) Hide published submissions (24)
Submissions
List of Papers (24) Toggle list
S2. Mechanisms of Action & Resistance
Session Chair
Prof. Dr. Jordi Vila, Department of Clinical Microbiology, Hospital Clinic, Barcelona, Spain
Show all published submissions (1) Hide published submissions (1)
Submissions
List of Papers (1) Toggle list
S3. Epidemiology & Multidrug Resistance
Session Chair
Professor Zhenbo Xu, School of Food Science and Engineering, South China University of Technology, Guangzhou, China
S4. Repurposing & Antimicrobial Adjuvants
Show all published submissions (3) Hide published submissions (3)
Submissions
List of Papers (3) Toggle list
S5. Antimicrobials & Interfaces
Session Chair
Professor Anthony William Coleman, LMI CNRS UMR 5615, Université Lyon 1, Villeurbanne, France